Last week I discussed that antagonizing glucagon has some significant downsides. Yes, glucose is lowered, but at a significant metabolic cost along with mechanism-based toxicity, so development of these drugs was stopped. However, in 2009 as these GCG antagonists were ramping up to start clinical trials, another group of scientists and physicians led by Richard DiMarchi and Matthias Tschöp proposed to instead agonize glucagon, an idea so radical that it was almost universally dismissed. In fact, DiMarchi gave a lecture at the European Association for the Study of Diabetes (EASD) in 2012 describing how to combine GLP-1 and Glucagon into a dual agonist single molecule. And 15 years later, it looks like they may have been right all along. Link to that video lecture below:
Before I go any further, I have to mention as a disclosure that a large part of my inspiration for doing this is because I was a phase 3 clinical trial participant for retatrutide. This drug is a next generation GLP-1, GIP, glucagon agonist being developed by Eli Lilly. I was on retatrutide for nearly 20 months, and its effects were so life altering I decided to start doing this educational blog in addition to working as a nurse practitioner full time. I have no other disclosures to make beyond this, but I felt it was important to maintain transparency.
So, let’s discuss what happens when you agonize glucagon instead. Yes, it'll raise glucose acutely, but that effect can be managed, especially if balanced out with GLP-1 and/or GIP agonists. Let’s also finish our biology review of glucagon’s actions that was started in the first article, but let’s expand our discussion about the hepatic actions of glucagon along with its pleiotropic effects IN and OUT of the liver and how much of glucagon’s other effects were known for decades, [1] but because of our inability to create long acting forms of glucagon, along with clinical bias about the glucose elevating effects, this blunted any further development and research into this area.
Image source: Hepatic glucagon action: beyond glucose mobilization | Physiological Reviews | American Physiological Society
Starting with the liver, and glucose production, why is the glucose rise so brief if you chronically agonize glucagon? In plain terms, you don't have unlimited glucose stores in your liver. A glucagon agonist does make your liver convert most of its glycogen into glucose, however, it also slows the production of new glycogen formation. Instead, glucagon will cause your body to enter into gluconeogenesis, that is the production of glucose from other substrates, namely, fatty acids and amino acids(aka fats and protein) So once that liver glycogen store is mostly depleted, you can push the lever on the glucagon receptor all day, but your sugar won't acutely rise nearly as much AND other benefits happen. I’ve highlighted a red bar around the section we’re discussing in the graphic below:
But if we were developing a drug, we still need to blunt the blood sugar rise, and surprise, glucagon itself is insulinotropic. Meaning it stimulates the release of insulin, especially when you are normoglycemic at baseline, but that leaves all our diabetic patients out in the cold. However, we can also recruit help from GLP-1(and GIP w/retatrutide) agonism as well. Both of these peptides potently stimulate insulin in a glucose dependent manner, and if you get the ratio of receptor binding correct, they will easily cancel the initial glucose rise.
What this means is biased multi agonist drugs would win out. Survodutide a GLP-1/Glucagon agonist has a binding ratio of 8:1 for GLP-1 to glucagon for example, it is actually a fairly weak GCG agonist. Pemvidutide is another GLP-1/GCG agonist with completely balanced activity, as such it doesn’t actually lower A1c, but many of the other metabolic effects persist. Mazdutide, yet another dual agonist, does not have published binding ratios but has potent GCG effects. Finally, retatrutide has a receptor binding ratio of 8x GIP, 0.7x GCGR and 0.6x GLP-1. With these 4 drugs we see a varied approach to cancelling out the glycemic effects. They all work to varying levels of success as we will review.
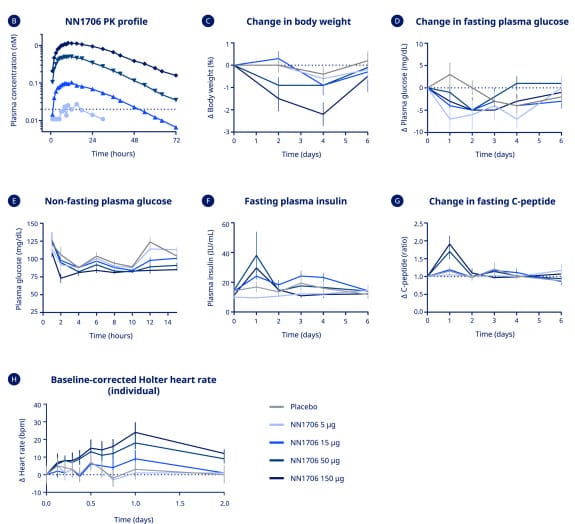
Finding the right balance of action is critical to developing a glucagon agonist
What's does this glycogen effect look like clinically? Hypothetically, it should cause a spike in insulin but only for a day or two at most. Thanks to Eli Lilly and Novo Nordisk we have phase 1 data from two different drugs. One of these drugs, retatrutide, is now in phase 3 trials, the other drug, NN1706 had development stop due to concerns over side effects, highlighting again that drug development is a difficult task. Anyways, with both drugs, we can see a very large insulin spike for ~2-3 days after a dose then it drops away and at the same time a very minimal glucose rise. [2, 3] Most likely what is happening is two-fold, one is that glucagon itself is insulinotropic, meaning it will increase insulin levels, especially in a non-fasted state. Second, is that the glucagon agonism of the drug is causing a large release of glycogen from the liver, this in turn further increases demand for insulin as a large influx of glucose enters the blood. After that, the liver doesn’t fully restore glycogen stores, but due to the glucagon agonism, gluconeogenesis will increase. Using the same data, we can also see with both drugs that glucose levels remain fairly stable, most likely due to the effects of GLP-1 and GIP helping to modulate glucose levels.
The next topic is fat, or rather lipids. Glucagon can be lipolytic. Meaning it'll burn fat(or in rather it’ll break down triglycerides into fatty acids and glycerol to be used for energy. You may hear this termed fatty acid oxidation or lipolysis. Regardless, it is one of the mechanisms of gluconeogenesis (the other is amino acids, more on that later) for creating energy from our energy stores.
This makes some sense in a prolonged fast or starvation you need to continue to mobilize energy to stay alive. As a personal opinion, I believe this might be one of glucagon's primary evolutionary benefits. Keeping you alive to find food. Looking at this very old graph (from a paper from 1970!), we can see as fasting progresses, insulin drops while glucagon stays high, and blood sugar is stable.
GCG agonism harnesses this fact. As noted above, it's decreasing your endogenous insulin production while your body “thinks” it's fasting from the GCG agonism, except you're not. You're still eating carbs. But you're burning fat too. In normal physiology this doesn’t happen, glucagon acts as a metabolic light switch, you can either burn primarily carbs or you can burn fats and amino acids, but not usually both at the same time. Think of a GCG agonist as that light switch getting stuck in the “ON” position and your body is the light bulb wasting light and heat when it’s not needed. Instead of conserving energy, glucagon is telling your body to burn excess fuel (calories) instead of storing them as fat or glycogen, in a sense this is a metabolic two for the price of one.
One way we can measure this effect is that fatty acid oxidation causes blood levels of ketones to rise, in a process called ketogenesis. In the chart below as presented by Eli Lilly in 2024, we can see their drug retatrutide causes a rise in ketone bodies. This effect was also seen in NN-1706, along with every other dual GLP-1/GCG agonist mentioned so far. Ketones are a preferred alternative fuel for the heart, brain and skeletal muscles and can also be used by the kidney for fuel as well. The chart shows ketones rising over 48 weeks of continuous retatrutide dosing. The light switch is stuck in the “ON” position with fatty acid oxidation and carb burning simultaneously! It’s like doing the keto diet, without actually having to follow a keto diet. In fact, as a personal anecdote, I would measure my blood ketones via a fingerstick while in my retatrutide trial and I would be in ketosis despite having eaten a carb heavy meal an hour before! This may explain some of the profound weight loss benefits seen with retatrutide in particular.
Image source: Eli Lilly presentation at ADA 2024
Because of this effect, triglycerides, and their cholesterol surrogate VLDL also drop rapidly in the blood. In the chart below from the phase 2 retatrutide trial we can see the triglyceride effect happens almost immediately in the first 8 weeks and continues to decrease without a plateau at 48 weeks. Across 3 drugs with GCG, retatrutide, survodutide & pemvidutide we see a 20-40% decrease in triglycerides & VLDL cholesterol depending on the drug.
And it isn’t just triglycerides. There is also about a 20% drop in total cholesterol & LDL cholesterol, with a neutral effect on HDL cholesterol. This is again down to the effects of GCG. It has action at both HMG-COA reductase (the enzyme that statins target) and at PCSK9 (the target for PCSK9 inhibitors) [4] It seems to also act on a complex called ANGPTL 3/8 which is thought of as sort of a master regulator of certain classes of lipids. [5] Without diving too deep (yet), glucagon action on these three sites help to drive down cholesterol and triglyceride levels.
Image Source: Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial | New England Journal of Medicine
This mobilization of fat also occurs in the liver, where glucagon potently stimulates hepatic fat oxidation. In English, if you have a fatty liver, then activating glucagon will rapidly clear liver fat. Both pemvidutide and retatrutide led to resolution of fatty liver in >75% of patients! Mazdutide and Survodutide both have trials ongoing for fatty liver disease and hepatic steatosis with positive results for both expected in the next year. Again, shown below is data from Eli Lilly for reductions in liver fat from retatrutide. Also, I will note that I am showing so much retatrutide data because Eli Lilly has been very open with the data, and it is also the furthest along in clinical trials.
Moving to weight loss, GCG agonism is apparently synergistic with GLP-1, since it is causing your body to burn excess adipose tissue and triglycerides, we may see more prolonged and potentially sustained weight loss with GCG drugs, but we need more data to confirm this. So far with survodutide, pemvidutide, mazdutide and retatrutide, there were no weight loss plateaus after 36-48 weeks of dosing the drug. Shown below is survodutide phase 2 data showing that on the 3 highest doses, patients were still losing weight after 46 weeks.
Looking at the kidney, retatrutide, and potentially survodutide (or glucagon agonists in general), seems to reduce blood pressure, we see an 8–14 mmHg drop in systolic BP depending on dose and drug studied. These drops in blood pressure, at minimum match the reductions seen with tirzepatide. Even more interesting, the blood pressure reductions shown by retatrutide in already hypertensive patients (>140 systolic) show a drop up to 30 mmHg, which generally exceeds the reductions seen with current commonly prescribed oral high blood pressure medications. [6] This is simply an extraordinary result and hopefully Lilly includes additional data for this effect in phase 3 trials.
Furthermore, retatrutide at least seems to preserve and potentially increase glomerular filtration rate (GFR) by almost 10 mL/min based on phase 2 obesity data. [6] Ongoing trials by Eli Lilly are studying this effect with a direct measurement of iohexol clearance GFR to see if it’s statistically and clinically significant, as this would be groundbreaking for the treatment of chronic kidney disease if confirmed. Results from the GFR study are expected at the end of 2025 or early 2026. This is again most likely a GCG-driven effect. The kidney has a large number of glucagon receptors, especially in an area called the macula densa. This very small collection of cells located just outside each glomerulus helps regulate glomerular flow and, by extension, blood pressure. This may explain both effects, but again, further data is required. [7, 8]
The GFR rise was most prominent in obese non-diabetic patients, and was not related to weight loss, fat loss or muscle loss. Diabetic patients saw a flatten GFR slope, and more data is needed to assess that particular group of patients
Finally, I want to circle back to glucose, after that initial dump of glycogen, blood sugar levels in diabetic patients tend to drop quite rapidly on these drugs. With up to 1.7% reduction with survodutide and nearly 2.2% with retatrutide, the reductions in A1c match available drugs such as semaglutide and tirzepatide. While it is easy to attribute this to the action of GLP-1 and/or GIP, the reality is actually somewhat more complicated. I want to quote from a research article here as it summarizes it better than I can:
“Acute glucagon action enhances whole-body insulin sensitivity independent from its insulin secretory effect, as well as independent from prior hyperglycemia and hepatic glycogenolysis in these experimental settings. Further, the improved insulin sensitivity was independent of GLP-1R and FGF21, but action through other receptors or other endocrine signals cannot be dismissed. Although paradoxical at first glance, it seems rational that since glucagon levels are elevated in a fasted state, which is a state of heightened insulin sensitivity, that glucagon is well positioned according to its physiological regulation to contribute to discrete aspects of insulin action as opposed to being an all-encompassing counterregulatory hormone to insulin. Thus, glucagon action improves glucose tolerance by amplifying insulin action in addition to the intra-islet paracrine effects to enhance insulin sensitivity.”
In plain terms, going back to my opinion on the evolutionary benefits of glucagon, the actions of glucagon are essentially priming the body’s pump for whenever we ingest calories again after prolonged fasting. This would allow for rapid metabolism and glucose clearance. But rather than a prolonged fast, we’re instead tricking our body into this state of heightened insulin sensitivity.
The reality of this is revealed again by referring back to phase 2 we have available. Survodutide and retatrutide both showed reductions in HOMA2-IR (a measure of insulin resistance), a drop in fasting insulin levels and perhaps most importantly, a very large reduction in the circulation of endogenous glucagon. If we go back to the glucagon antagonists for a moment, the goal was to block the actions of glucagon and thereby allow insulin to work more effectively. In an ironic twist by agonizing glucagon, we achieve this effect far more effectively than any drug currently on the market. To put a number on this, in phase 2 data, retatrutide decreased plasma glucagon levels between 60 to 89% Fasting insulin levels dropped 25-40%, and these reductions happened within the first 6 months of first dose administration! The metabolic turn around is quite rapid on these drugs.
Now I’ve put a lot of positives down, but for a moment I have to absolutely discuss the negatives and there are two potential hurdles that glucagon agonists have to clear. One is relatively easy; the other is less so.
The first is that glucagon is a catabolic hormone. Going back to burning fats, glucagon’s role is equivalent to burning whatever excess fuel you have in your body to keep you alive. We’ve discussed carbs and fats, but I haven’t mentioned proteins, or more accurately amino acids. Stimulating glucagon also increases the turnover rate of amino acids in the liver. Again, referring back to the current drugs in trials we see reductions in plasma amino acids up to 40% in phase 2 data. [9] Put another way, if you run out of fats to burn, glucagon and your body start going after proteins, like burning the blankets to stay warm in the winter. This could be very counterproductive if a person doesn’t eat a diet that has a fairly high amount of protein and does some sort of resistance training. In essence, while this could be very problematic, it is easily solved through the old standbys of exercise and a proper diet but will require vigilance on the part of patients and providers.
The other problem is heart rate increases. In all the trials of all GLP-1 medications heart rate elevations were noted, usually by a few beats a minute. GLP-1 causes an increase in heart rate through a direct mechanism we won’t discuss here. Glucagon also causes an increase in heart rate when given at pharmacological doses. Combine the two into one drug and you get an additive effect. In all of the available data from the drugs mentioned heart rate increased somewhere between 6 and 10 beats per minute on average. However, some patients may see increases up to 20 BPM. This effect may also be confounded by the reductions in blood pressure mentioned earlier, when blood pressure is rapidly dropped, our heart rate compensates to keep blood flow rates the same. This can be seen as a potential problem requiring monitoring. The good news is the effect appears transient. After about 6 months heart rates start returning to normal in all the available data. I don’t think this will derail regulatory approval, but it may require closer monitoring by doctors and consideration on who can receive one of these drugs.
To summarize, the glucagon agonists are literally opposite the antagonist, which is what you’d hope would be the case! These drugs lower cholesterol, triglycerides, glucose, weight, reduce liver fat, lower BP, protect the kidney and potentially more! I didn’t even mention that these drugs also reduce uric acid levels and therefore may have a role for gout as well. They may reduce the risk of arteriosclerotic cardiovascular disease with its dual weight & lipid lowering effect?? Will they usher in a new era of kidney disease treatment if they really do increase GFR? Will we finally have an effective cure for fatty liver disorders?
We won’t have to wait much longer to start discovering answers to these and many more questions, but I hope you can see that despite its reputation, glucagon isn’t just the counter regulatory hormone to insulin.
It’s a regulatory hormone full stop.
It is critical to multiple metabolic pathways and even with this article pushing 3500 words, I could easily write another 5000 on how it is critical to our metabolic health. But I think the point is well made. Despite decades of research on how to reduce glucagon, block it, or otherwise try to get rid of it, the answer is all along with to activate glucagon instead and harness its metabolic powers to usher in a new revolution in medicine. GLP-1 mono-agonists were the start of this, adding glucagon to that class of drugs will for now represent a triumph of medicine in my mind and I am excited to see what comes forth from all the clinical trials that are ongoing right now.
My next entry will be highlighting new and interesting presentations from the ADA 2025 conference so stay tuned for that in a few weeks and feel free to share this blog! I’ve included an extensive number of references and further readings, because as I noted, there is so much more!
References and further reading:
