🌟 Editor's Note
This will be part two of multiple parts covering all the announcements at the American Diabetes Association 2025 scientific conference, the first article was on CagriSema, this will cover Amycretin and MariTide
Link to the CagriSema article: ADA Conference 2025 review: CagriSema
Amycretin Phase 1b/2a trial & MariTide phase 2 trial
Amycretin trials: A phase 1 safety/tolerability trial and then a phase 1b/2a trial in subjects with obesity
MariTide phase 2 trial: Obese non-diabetics and obese diabetics in one combined trial.
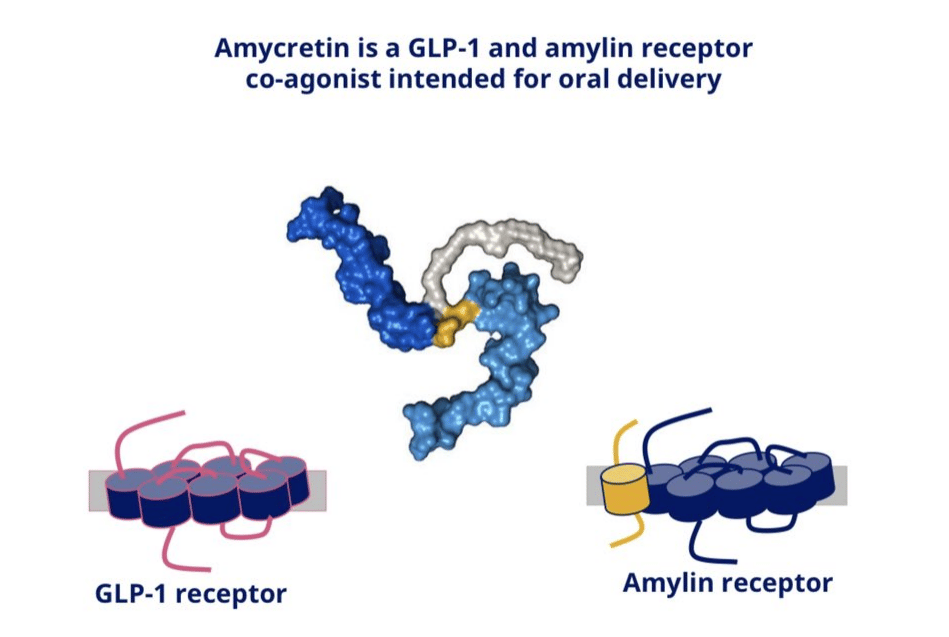
What’s Amycretin & why it matters
Similar, but better version of CagriSema?
Yesterday we discussed CagriSema, which is two separate molecules, one amylin, one GLP-1 and in order to be injected they must be kept separate as combining the two basically make peptide based super glue. In continuing with Novo’s big bet on Amylin, they managed to make a unimolecular Amylin-GLP-1 dual agonist. The structure of which is the thumbnail for this article. On one side is a GLP-1 agonist, in the middle is a glycine linker with a fatty acid backbone for half-life and stability and then the other half is the Amylin agonist. The other way to view is like this:

Why create the same drug twice?
As mentioned, CagriSema requires keeping the drugs separate until the last possible moment, this requires a dual chamber injector which is expensive and requires creating two molecules. Amycretin solves that problem, one molecule, single chamber injection OR as an oral pill, in addition changes were made to both the Amylin and GLP-1 structures. Arguably, besides CagriSema this was the drug that many folks were excited about. But then the data was published in the Lancet.
Behind a paywall.
Setting aside that paywalls are annoying and anathema to the progression of knowledge, it is yet another way to put your best foot forward safe in the knowledge that many won’t try to get around the paywall. Fortunately, I have access.
Massive weight loss…but side effects
After 36 weeks, once-weekly subcutaneous amycretin up to 60 mg reduced bodyweight by 24% compared with a 1% reduction with placebo.

At first blush, these results are actually spectacular for the 60mg dose of Amycretin. These match the weight loss seen with retatrutide but in a shorter timeframe! Even the smaller doses caused significant weight loss between 15 and 20% for the 5 and 20mg doses respectively. However, the side effects were worse than what we saw with CagriSema. If we compare with pooled side effect data from the retatrutide phase 2 data vs pooled amycretin data, it becomes pretty clear there is an issue here.
Retatrutide pooled side effects
Nausea 27%
Vomiting 10%
Constipation 9%
Diarrhea 13%
Amycretin SQ pooled side effects
Nausea 57.5%
Vomiting 29.5%
Constipation 15%
Diarrhea 20%
I can’t make that much more obvious in terms of tolerability. Now I mentioned that Novo had made an oral formulation of Amycretin and the story is arguably no different in that trial for side effects. In terms of other benefits there were better reductions in cholesterol than were seen with CagriSema. But at the end of the day, Novo again has a molecule that needs work in terms of tolerability. They will be starting phase 3 trials of Amycretin soon with rumors of smaller doses and longer dose titration. I’m not sure even that can save it as the rates of side effects were higher on the 1.25mg and 5mg dose than anything that is currently FDA approved. Exciting, but caveats again. Now what about MariTide?
MariTide, antagonize GIP, antagonize your GI tract
Maridebart Cafraglutide aka MariTide is being developed by AMGEN, so finally a company that isn’t Eli Lilly or Novo, is a GLP-1 receptor agonist attached to a monoclonal antibody(MAB) that is a GIP receptor antagonist. It is a first in class drug, not only because it’s a MAB, but because of the GIP antagonist action. Eli Lilly believe that GIP agonism is the correct path to take, and weirdly for drug development both pathways work to increase weight loss but that will have to wait for another post. The other unique action of MariTide is once monthly dosing instead of once weekly. So how’d it do?

It actually did really well! For both obesity and diabetes there were large reductions in weight, and for diabetics up to 2.2% decrease in A1c. Blood pressure reductions were up to 10mmHg systolic. Lipid changes were equivalent with tirzepatide with statistically significant reductions in triglycerides, LDL and VLDL cholesterol. But once again the side effects are about to make this ugly.
The downside of once a month medications in a nutshell
What if I told you that a drug I could prescribe you would give you a 33% chance of vomiting. Would you take it? What about a 70% chance? What if I told you no matter what dose of this medication I gave you, you’d have a 60% chance of nausea. Well that’s exactly what happened with MariTide. And guess what? The half life is 3 weeks so I hope you’re stocked up on Zofran.
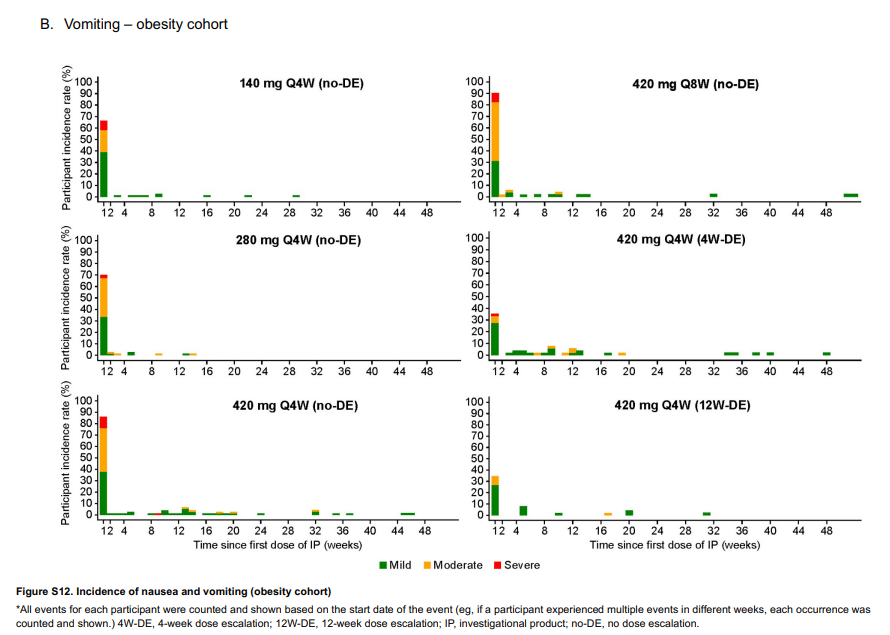
The reasons for this are multiple. For one, I will just call out Amgen. Every single trial of GLP-1 medications has found that the best way to avoid side effects is a slow titration. Amgen instead asked, how about ZERO dose titration and gave some patients the maximum dose with NO titration and the results speak for themselves. Even with the slow titration(bottom right) there was STILL a 30% vomiting rate. I will flat out say the weight loss is impressive, especially for the diabetes arm, but clearly some of this weight loss is mediated by the amount of vomiting that happens in the first month. Thankfully for Amgen, after the first month it goes away, probably due to tachyphylaxis, otherwise this drug would be dead on arrival.
Anger the area postrema at your own risk
The brain’s vomiting center is neither shy or coy
However, that brings me to my next point and it’s relevant for Amycretin, CagriSema and MariTide. GLP-1, Amylin and GIP all work in the brain as well in a slice of the brain called the area postrema. If you’ve had medical training you might know this as the vomiting center of the brain. Amylin and GLP-1 both activate neurons in the area postrema in a way that at first blush from trial data seems synergistic given the higher rates of nausea and vomiting.
But here’s the interesting caveat, GIP agonism actually works to chill out those neurons. As in, it has an anti-nausea and anti-vomiting effects. Eli Lilly has actually tested this out by giving patients a GIP receptor agonist, allowing it to concentrate in their blood then giving them a GLP-1 agonist the next day, and it worked! Patients had less nausea and less adverse events overall.
Now, enter MariTide. It is a GIP antagonist AND a GLP-1 agonist. So just like amylin and GLP-1, you now have the area postrema being over stimulated and in the end there’s vomit all over the place.
That being said Amgen, has committed to much lower starting doses for the Phase 3 trials of MariTide, and a low and slow titration just like everyone else. Given the fact that the nausea and vomited was really limited after the first month of dosing I actually have hope that Amgen may just figure this out and we’ll have a viable 3rd option in a few years.
We still don’t know where it would fall in terms of absolute weight loss as no plateau had been reached, but regardless it would be yet another option and from a different company.
To wrap up today, I’m extremely neutral on Amycretin as Novo absolutely needs to get the side effects under control or else, I will repeat myself from yesterday, who am I going to prescribe this to with such high rates of GI side effects.
I am more hopeful for Amgen and MariTide as they seemingly have learned their lesson about why we slowly increase the dose on GLP-1 medications, and it could easily find a place given the ease of once monthly dosing, especially for a diabetic patient.
Final installment tomorrow: Orforglipron, bimagrumab, and finally some lower side effects
References: