🌟 Editor's Note
This will be part one of multiple parts covering all the announcements at the American Diabetes Association 2025 scientific conference, up first CagriSema
CagriSema Phase 3- Redefine 1 & 2 trials
Redefine 1- A phase 3 clinical trial of a combo of cagrilintide and semaglutide in patients with obesity.
Redefine 2- A phase 3 clinical trial of a combo of cagrilintide and semaglutide in patients with obesity and type 2 diabetes
What’s CagriSema & why it matters
Amylin agonist + GLP-1 agonist
Building off the success of Ozempic/Wegovy, Novo Nordisk created a new molecule based off amylin, called cagrilintide. They then decided to trial it with semaglutide which is the active molecule in Ozempic/Wegovy.
But what’s Amylin? Well amylin, like GLP-1 is secreted from the pancreas, in this case it is co-secreted with insulin at a ratio of 100:1, that is for every 100 molecules of insulin, 1 molecule of amylin is secreted. Like GLP-1, amylin is thought to slow gastric emptying, reduce glucagon secretion, increase satiety and reduce appetite.
Novo Nordisk bet big on this combo
The idea of this combination would be synergistic together to lead to more weight loss than either peptide could produce alone, and that potentially could lead to better glucose control for diabetic patients. Novo also claimed that the combo could reach 25% weight loss for those on the highest dose, which would be greater than the weight loss seen on Tirzepatide(Mounjaro/Zepbound) Were they right?
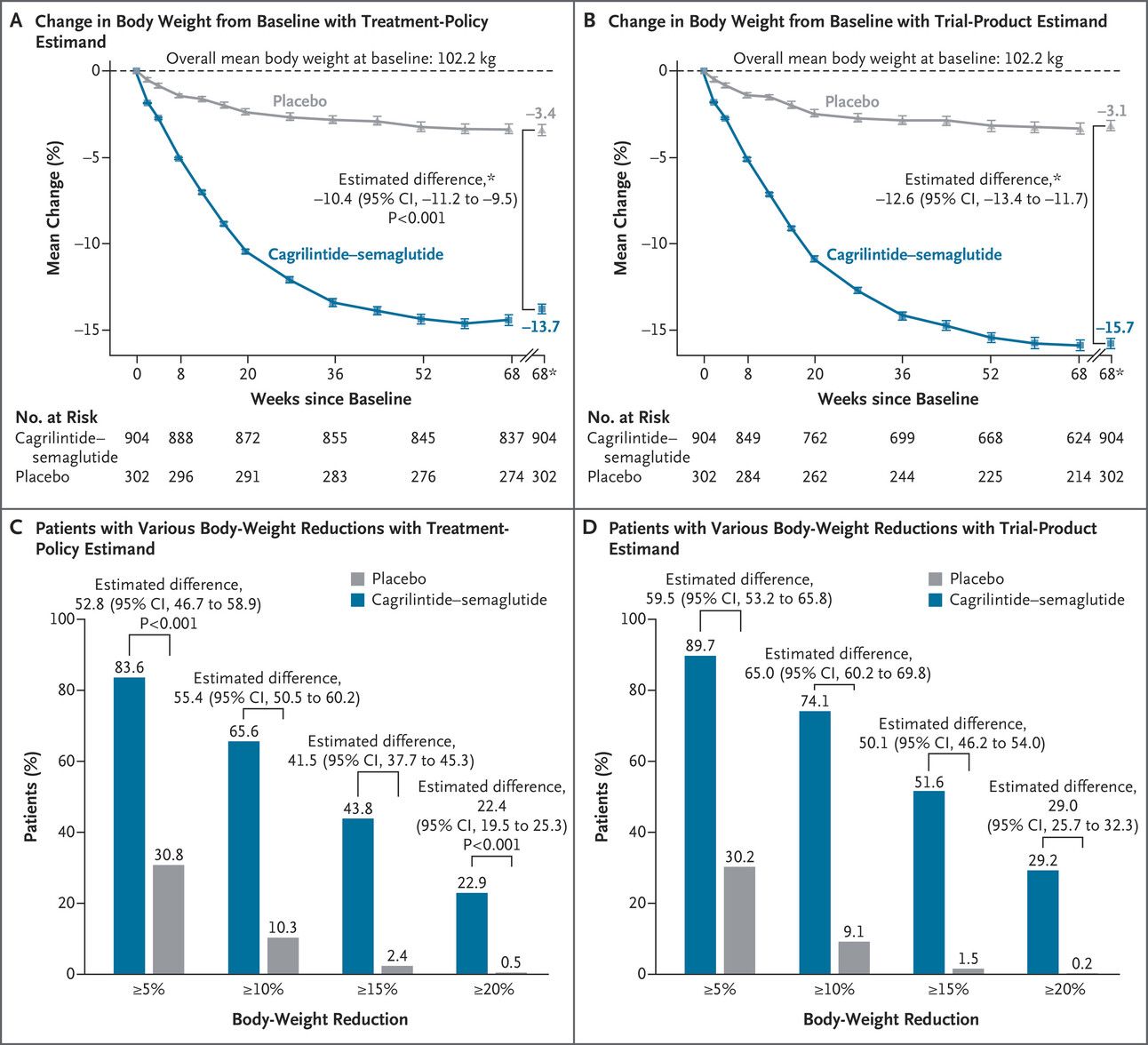
Overpromised & slightly underdelivered
On the basis of the trial-product estimand, the percent weight reduction was −22.7% with cagrilintide–semaglutide combo

In a word. No. They missed on the weight loss target. I suspect, this was for a few reasons, the first of which was the trial itself was too short at only 68 weeks as you can see on the graph that the weight loss had not entirely plateaued. The other reason is that Novo allowed flexible dosing, that is, they allowed patients to dose escalate up or down if the patient wished to do so. That’s great for tolerability, and more realistic to how dosing happens in the real world, but not great when you’re expecting to hit a certain number. The final reason is that this drug combo does not increase insulin sensitivity, and without that final key, I personally believe that is a minor contributing factor as well, more on that in a moment with the diabetes data. To Monday morning quarterback, if this trial were at least 80 weeks long, with fixed dose escalations up and flexible dose titrations down as needed for side effects, then I bet I’m writing a very different article about CagriSema being the second drug to ever hit 25% weight loss on average. (Tirzepatide was the first)
But what about the diabetes trial?

This was the more promising of the two trials in my mind. The reductions in A1c and weight loss essentially matched tirzepatide blow for blow. There was a clear plateau in weight loss, but I suspect as I noted earlier that if this drug actually helped with insulin sensitivity the weight loss may have been greater.
Non-scale benefits, AKA, the cardiometabolic benefits
In both trials reductions were noted in blood pressure, around 10mmHg systolic for Redefine 1, and about 6mmHg systolic for Redefine 2, which is generally a little bit better than semaglutide alone.
In both trials participants had improved quality of life across almost every metric tested as one would expect when you lose significant amounts of weight and have control of your diabetes.
And to put my own caveat in place for what I’m about to say next, greater weight loss is a benefit in itself, reaching 15-20% weight loss does allow for reversal of such things as sleep apnea or remission of Type 2 for example.
But I hate to disappoint my readers, but that’s it on further benefits when compared to semaglutide alone
The caveats, AKA the side effects and downsides
Don’t mind the drug behind the curtain supplementary appendix
As I go forward with this series, I want to make one thing clear, while we don’t have many direct comparisons available, using past clinical trial data to indirectly compare is a valid strategy and one we must keep in mind as this deluge of information comes forth. Finally, let’s keep in mind something important. Weight loss IS important, but it should NOT be the most only important thing we evaluate. And as a mentor once told me, always read the supplementary appendix.
One curious thing that an attentive reader might notice is that the adverse events/side effects in the main body of the paper is…lacking detail. In both papers there is a generic label for “gastrointestinal disorder.” There is no breakdown by nausea, vomiting, diarrhea or constipation. Instead, you’ll find both of those things buried in the supplementary appendix. For ease of viewing, I’ve snipped both.

Redefine 1 side effects
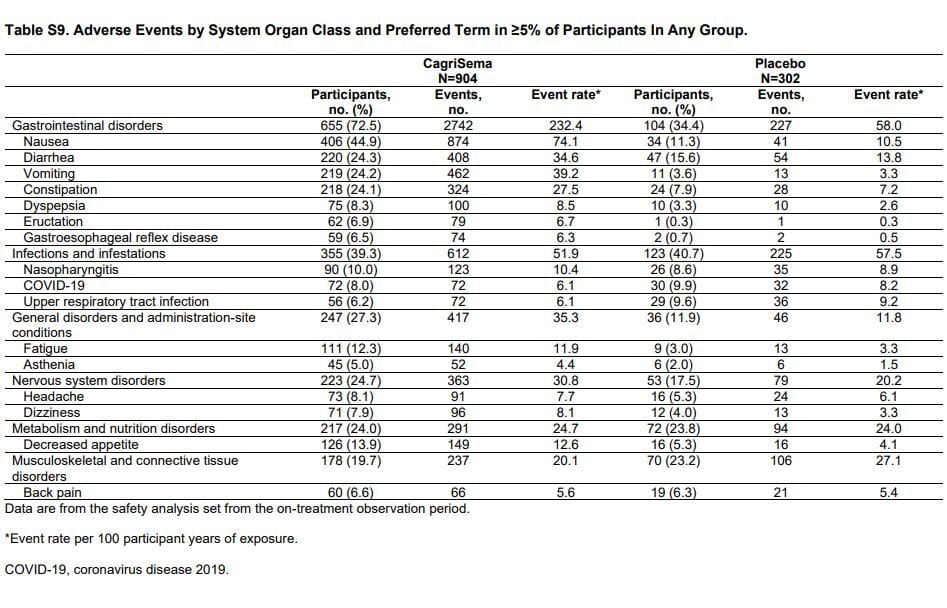
Redefine 2 side effects
The immediate thing that leaps out is that CagriSema has more side effects, and especially more nausea. But don’t look at the percentages, they’re higher but don’t tell the full tale. Look at the raw number of events. Staying with the nausea for a moment, there were approximately 2700 incidents of nausea on CagriSema and 270 on semaglutide alone, but there were seven times more patients on CagriSema. If we extrapolate the semaglutide data to assume the same number of patients as CagriSema, there would be 1900 incidents of nausea. That’s not good. The same holds for all the other GI side effects.
The diabetes trial requires a bit more digging, but I went back to the SUSTAIN 6 trial of semaglutide and I’ll spare you another chart of side effects, but the story is the same. Substantially higher rates of GI side effects, nearly double the amount of nausea for example.
It does not get much better from there for other cardiometabolic benefits. The changes in triglycerides and cholesterol were no better than semaglutide. As noted earlier, there was no significant change in measures of insulin sensitivity (HOMA-IR) The changes in fasting insulin were similar.
Now I am not one to slander a multi-billion-dollar company, but I will simply say that if you have to put this information into the appendix and not the main body of your paper, then I assume that is the company’s acknowledgement that a problem exists.
Is higher weight loss alone worth the extra side effects?
A pattern that will repeat itself
Let’s summarize this, CagriSema both for diabetes and obesity is essentially as effective as tirzepatide for weight loss and A1c reductions. Otherwise, it is not nearly as tolerable as semaglutide alone, and especially not tirzepatide. It offers no appreciably greater cardiometabolic benefits than semaglutide alone. This will be a pattern that emerges as I review Amycretin, also by Novo, and MariTide by AMGEN in the next article.
As someone who prescribes these medications, I am torn. I am happy to have another option to provide to patients, but also who exactly am I going to prescribe this to? In my mind, I imagine I’ll be writing Zofran at the same time that I’m prescribing this medication, which I generally don’t have to do with tirzepatide. If history is a guide in the pharmaceutical industry, then the drug with less side effects nearly ALWAYS wins out in the end. Novo has started more phase 3 trials with CagriSema to try and show it can hit that 25% weight loss. With that in mind, I am not sure what else to say. By the time those longer CagriSema trials are finished, all the phase 3 trials for retatrutide will have wrapped and that drug is even MORE potent than either tirzepatide or CagriSema.
Next up- Amycretin and MariTide, the side effects strike back
References:





