🌟 Editor's Note
This is part three of multiple parts covering all the announcements at the American Diabetes Association 2025 scientific conference
ACHIEVE-1 phase 3 trial & BELIEVE phase 2 trial
ACHIEVE-1 A phase 3 clinical trial of orforglipron monotherapy in early Type 2 Diabetes with obesity
BELIEVE A phase 2 trial of combination bimagrumab & semaglutide on weight loss and lean mass preservation
What’s Orforglipron & why it matters
Non-peptide based, traditional small molecule GLP-1 agonist
I must confess, my first two posts about the ADA conference slanted negative. Today’s final post will at least be more positive for one of the drugs! Let’s start with orforglipron. This is a pure GLP-1 monoagonist drug, however, it is NOT a peptide. Semaglutide, tirzepatide, anything that ends in -tide is a peptide, meaning it’s constructed of amino acids. This also means that if you were to ingest them as an oral medication, enzymes in your gut would rapidly break them down and they would have no effect.
Novo Nordisk has an oral formulation of semaglutide branded as Rybelsus, however, this requires a proprietary additional molecule to protect the peptide from digestion, however, its bioavailability is poor and it requires special dosing, it must be taken first thing in the morning with a small amount of water, and then nothing else can be eaten for at least 30 minutes after the dose. Because of this, patient use of this medication has been slow at best.
Orforglipron looks to change all of that. Being a ‘traditional small molecule’ means it is relatively easy to mass produce, requires no special dosing and has good bioavailability. Eli Lilly is heavily invested in this medication as they seem to be of the mind that this medication can be an alternative to injectable GLP-1 meds and/or maintenance medication after weight loss in high income countries and allow for easy distribution to the rest of the world and give access to a GLP-1 medication for the first time. Importantly, it should also be cheaper.
This first trial, ACHIEVE-1, is essentially a proof-of-concept phase 3 study to show that orforglipron alone could achieve sustained reductions in A1c and weight. The patients were patients that mostly had never been on a medication for diabetes before, had an A1c between 7 and 9.5% and had been trying diet and exercise to control their diabetes. These are patients you see all the time in primary care, a newly diagnosed diabetic, currently guidelines recommend metformin as first-line, so how did orforglipron do?
Metformin but better?
Mean glycated hemoglobin level at week 40 was 6.5 to 6.7% with orforglipron (it works!)

Comparing the 3 doses to metformin monotherapy reveals that orforglipron is better at 12mg and 36mg and essentially matches metformin at the 3mg dose. Nearly 75% of patients had an A1c under 7 in just 40 weeks and blood glucose control and glucose excursion were significantly improved on it. This also is in line, and just a little bit below the A1c reductions of 1mg of injected semaglutide.
And how’s the weight loss and metabolic markers?

It was nothing remarkable, about 8% for the highest dose but essentially matched the weight loss that a diabetic patient would see on 1mg of injectable semaglutide. Interesting, maximum weight loss had not been achieved at 40 weeks, and we will have to wait for longer duration trials which should publish by the end of 2025. Also importantly, there were reductions in lipids, again nothing major, but in line with other GLP-1 monoagonists. Blood pressure reductions were also noted of about 6mmHg systolic.
And the side effects….
For once they’re good! Or at least, in line with expectations for other GLP-1 medications, I am using pooled rate for side effects and in order by percentage:
Diarrhea 18.6%
Dyspepsia (GERD) 13%
Nausea 12%
Constipation 11%
Vomiting 7%
This is much more in line with what you would like to see from a drug in phase 3 trials. A slight damper to note is that while these rates were low, the nausea and constipation seemed to be much more persistent for a subset of patients. Most patients on GLP-1 meds experience tachyphylaxis, that is the body essentially adjusts to the med and the side effects diminish. In comparison, while diarrhea was the most common complaint, the event rate diminished with time as we usually expect. This will be something to keep an eye on in the longer trials that will be coming later this year.
To wrap this section up, orforglipron looks very promising overall as a drug. It is cheap and easy to make, seems well tolerated, is as effective as injectable GLP-1 medication, provides better weight reduction and A1c reduction than metformin alone. I look forward to the 5 other trials that will be presenting data on this medication in 2025, especially the diabetes trials when it is combined with metformin and/or SGLT2i drugs. Most importantly, it will allow for mass distribution of a GLP-1 medication worldwide at a time when Type 2 diabetes rates continue to rise.
So, with that settled, onto our final and perhaps one of the more novel medications presented at ADA
Bimagrumab, lose fat, save muscle & strength?
Just don’t look at the cholesterol numbers?
Bimagrumab. Just rolls right off the tongue, doesn’t it? This one is a bit wild, and it appears effective but there is one single lingering question. Like MariTide this is a monoclonal antibody, but it is not an incretin. Instead, I’ll let this slide handle the explanation:

Without going crazy deep on the science, it essentially helps increase/preserve muscle mass while actively decreasing fat mass/fat storage. The result is rather remarkable, in earlier studies bimagrumab monotherapy caused 100% fat loss and 0% lean mass loss. In the final twist, Eli Lilly owns this antibody, but acquired the company that developed it AFTER that company had started another phase 2 trial with semaglutide. So, Eli Lilly is presenting data on semaglutide. Weird. Also forgive the screenshots, as this was presented on a slide deck and I’ve not been able to gain access to the slide deck so far and Lilly has not published this data in a journal yet.

The really good stuff
This trial had NINE arms, but most of the slides focus on these 3 arms, Bima 30mg/kg, Sema 2.4mg or a combo of Bima 30mg/kg + Sema 2.4mg. As we can see the combo of the two significantly increased weight loss, essentially to the level one would see with tirzepatide, with only 3% lean mass loss according to a DEXA scan. The Bima monotherapy actually led to INCREASED lean body mass and that magical 100% fat loss! This helpful chart was found on Twitter showing just how much Bima actually preserved lean mass while increasing weight loss.
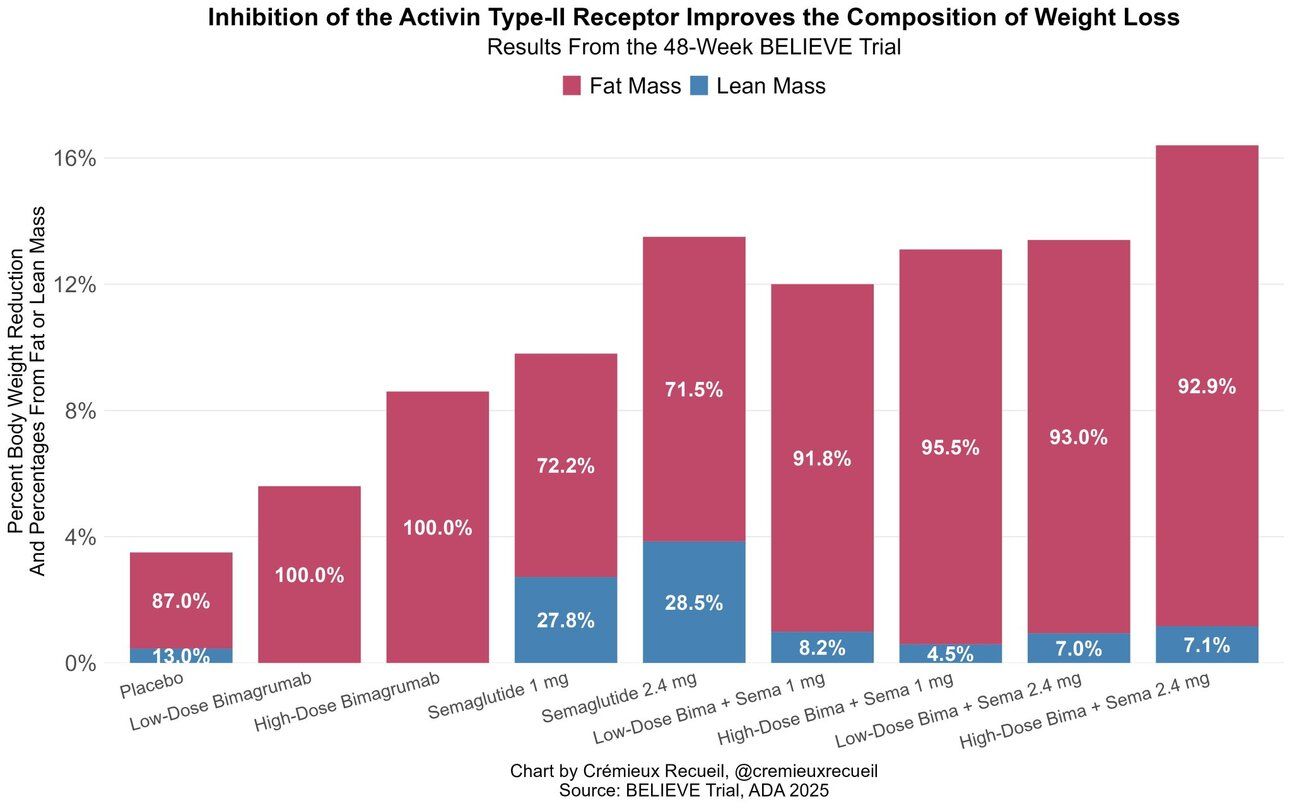
However, I noticed something that was presented that will require further data and a more detailed look at the actual trial, hopefully when it is published in a journal.

Can you spot the problem? The 17% increase in LDL cholesterol and the fact that it wipes out pretty much all of the cholesterol and lipid reductions that semaglutide alone provides. That needs to be immediately figured out or this drug will be dead on arrival at the FDA as it would be potentially too risky to give to someone with cardiovascular disease. Interesting, in earlier trials the lower dose of bima at 10mg/kg had no effect on lipids whatsoever. More data, more trials needed, and thankfully Eli Lilly is doing just that, but put a giant asterisk next to bima for now.
Lastly, I have to ask what I asked of CagriSema, who is this drug for? Sarcopenic obesity patients? Obese but frail patients? I’m not sure nor convinced yet. My other question is this: Are people losing functional strength on GLP-1 medications? I’m sure some are, but would simple resistance training be enough to preserve it? I have many questions about bima that are left unanswered, especially on cholesterol. Is there a dose that preserves lean mass without the lipid liability?
While I could probably turn this into a 10-part series, I’m going to leave it here. The information presented at the ADA 2025 conference was basically the equivalent of a massive waterfall. There were numerous other incretins presented that may or may not become more relevant over the coming years. If I were to recommend a couple, take a look at NA-931, HDM1005 and pemvidutide. There was a presentation on a stem cell therapy that essentially cured Type 1 diabetes in 10 out of 12 patients. There was data on lipids, blood pressure and so much more, and I’d encourage you to seek it out on your own.
My next work in progress will be on GIP and why it is the most important incretin despite GLP-1 getting all the airtime at the moment. Stay tuned!
References:


