Breaking Metabolic News (and bariatric surgery barriers)
Link to the press release: Lilly's triple agonist, retatrutide, delivered weight loss of up to an average of 71.2 lbs along with substantial relief from osteoarthritis pain in first successful Phase 3 trial | Eli Lilly and Company
You know, I was ALMOST done with my GLP-1 and kidney blog. Like I planned to finish it this weekend and publish it on Monday next week. Then Eli Lilly has to go ahead and drop a dang bombshell and interrupt my plans.
So instead, here’s a quick run-down and initial thoughts on TRIUMPH-4 topline results which posted today.
So first the trial and I’m just going to cut and paste from Lilly’s pressor on this part as to what the trial actually is:
TRIUMPH-4 (NCT05869903) is a Phase 3, 68-week, randomized, double-blind, placebo-controlled study comparing the efficacy and safety of retatrutide with placebo in adults with obesity or overweight and knee osteoarthritis. The study randomized 445 participants in a 1:1:1 ratio to receive either retatrutide 9 mg or 12 mg, or placebo. The objective of the study was to demonstrate that retatrutide is superior to placebo in WOMAC pain subscale score reduction and in body weight reduction from baseline to week 68 in people with a BMI ≥27.0 kg/m² and who met American College of Rheumatology Criteria (clinical and radiological) for knee osteoarthritis. Participants randomized to retatrutide initiated treatment with 2 mg once weekly and increased the dose in a step-wise approach every four weeks until reaching the target dose of 9 mg (via steps at 2 mg, 4 mg and 6 mg) or 12 mg (via steps at 2 mg, 4 mg, 6 mg and 9 mg).
Nearly 450 patients, 1/3 on placebo and 1/3 on 9mg retatrutide and 1/3 on 12mg retatrutide and the results are nothing short of astonishing:
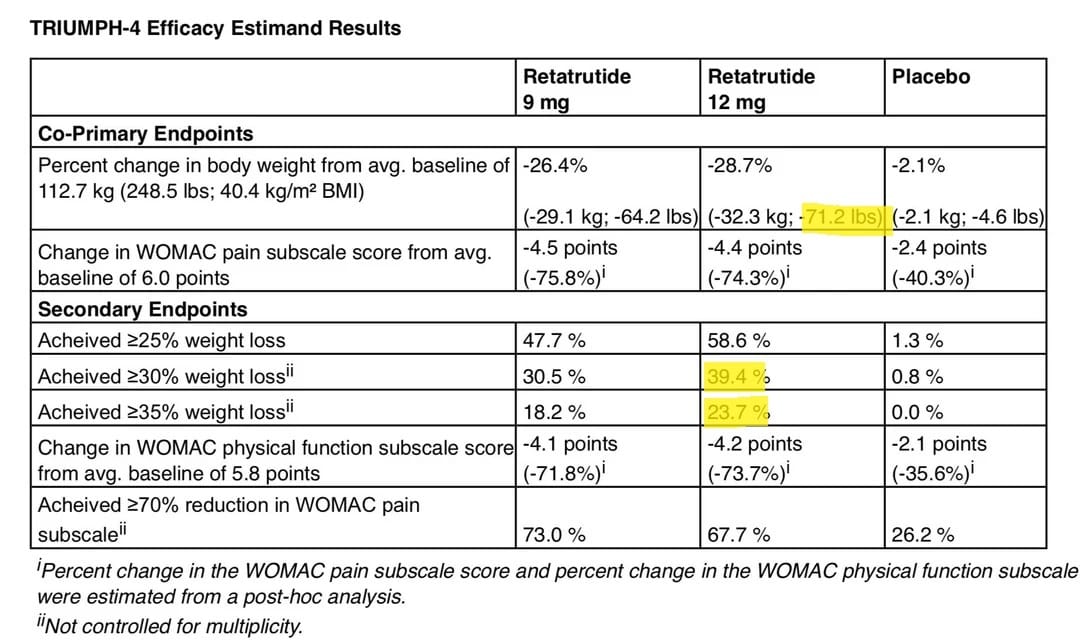
Now I’m going to talk about downsides, but let’s just sit with this for a minute. We are just shy of 29% weight loss in only 68 weeks as the average. That’s an average of 71 pounds. Even more than that nearly a QUARTER of patients lost over 35% of their starting body weight. That is absolutely insane.
Bariatric surgery whether gastric sleeve or Roux en Y gastric bypass generally expects patients to lose 25-35% of their starting weight. This result is square in the middle of that range AND Eli Lilly cautioned a few months ago that these results ‘will not show the true efficacy or final weight loss potential.’
So, uh. Whoa.
For further comparison, while Novo Nordisk didn’t publish >30% weight loss the recently released STEP-UP trial which looked at semaglutide 7.2mg vs 2.4mg for weight loss. They only reported >25% weight loss. On 7.2mg dose 33% loss that much, and only 17% lost that much on 2.4mg. This trial blows both of those results out of the water.
How about Tirzepatide? The Surmount-5 trial had 25% and 30% weight loss reported, and max tolerated dose of tirzepatide caused 36% and 22% of patient hitting those two marks respectively. What about CagriSema? 35% and 19% Same story here, it’s closer but still far and away more weight loss.
Secondarily, this trial was for knee osteoarthritis, and to see if retatrutide could help that, and the answer was unequivocably yes. There was a consistent reduction in pain using the WOMAC pain subscale and about 15% of patients reported being pain free by the end of the trial. While that may not seem like many patients, keep in mind osteoarthritis is a degenerative disease and retatrutide is not a pain medication.
Other minor data points made by Lilly included that patients on retatrutide had an average systolic blood pressure reduction of 14mmHg. That is about double what we see with tirzepatide, nearly three times better than semaglutide and 4mmHg better than the CagriSema trials. So potent anti-hypertension effects as well, presumably driven by such excessive weight loss. We’ll learn more about that when they actually publish the trial data.
The downsides (aka side effects)
Here’s where I get to pop the excitement balloon.
Consistent with the types of adverse events seen in clinical trials for other incretins, the most common adverse events among participants treated with retatrutide (9 mg and 12 mg, respectively) were nausea (38.1% and 43.2%) vs. 10.7% with placebo, diarrhea (34.7% and 33.1%) vs. 13.4% with placebo, constipation (21.8% and 25.0%) vs. 8.7% with placebo, vomiting (20.4% and 20.9%) vs. 0.0% with placebo, and decreased appetite (19.0% and 18.2%) vs. 9.4% with placebo. Dysesthesia occurred in 8.8% and 20.9% (9 mg and 12 mg, respectively) of patients treated with retatrutide, compared to 0.7% with placebo. These dysesthesia events were generally mild and rarely led to treatment discontinuation.
I’ll be blunt and flat out; these are not the best side effects I’ve ever seen but also not the worst. I’m going to use the SURMOUNT-5 data again as a comparison in which case the nausea rate is the same, the constipation rate is slightly better, the diarrhea rate is slightly worse and the vomiting rate similar to semaglutide but worse than tirzepatide. Comparing to CagriSema, it better tolerated in all GI side effects except diarrhea.
The real key is going to be side effects over time, if retatrutide is like other drugs in the class then the side effects should decrease with time, but unfortunately, we don’t have that today, at this point I have some questions and minor concerns but without the data over time it’s hard to speculate about tolerability further beyond saying it’s similar to other drugs in the class.
But what the hell is dysesthesia you ask? In more basic terms you’ll hear more patients say its skin sensitivity, a mild burning/tingling sensation, sensitivity to certain fabrics or temperatures on their skin. Going back to the STEP-UP trial with semaglutide it was seen in about 20% of patients in that trial as well on the 7.2mg dose. We don’t know the cause yet, and in both trials, it apparently led to a few patients stopping the drug. There have also been anecdotal reports of patients experiencing this on tirzepatide as well. Not necessarily a big concern, but if a potential treatment or cause for this can be discovered, I presume that would be helpful.
Continuing on to discontinuation rates we see another very interesting nugget that I have bolded below:
Discontinuation rates due to adverse events were 12.2% and 18.2% with retatrutide 9 mg and 12 mg, respectively, compared to 4.0% with placebo. These rates were highly correlated with baseline BMI and included discontinuations for perceived excessive weight loss. For patients with a baseline BMI ≥35, discontinuation rates due to adverse events were 8.8% and 12.1% for the 9 mg and 12 mg doses, respectively, compared to 4.8% with placebo.
At first, I was shocked to see such a high rate of patients stopping the trial, but then that second line is doing alot of heavy lifting. Eli Lilly, if I’m reading this right is suggesting that somewhere between 4-6% of patients in the retatrutide arms stopped taking the drug due to excessive weight loss. (Note: I arrived at that number by doing some quick back of the napkin math based on 84% of the patients have a BMI>35 for this trial as reported by Lilly)
If my math is right about 71 patients had a BMI < 35 and Eli Lilly is suggesting that group in particular was stopping due to excessive weight loss. If that’s the case Lilly is suggesting something like 20-25% of that group lost too much weight. I’m purely speculating off some rough math, but that would be incredible and would go a long way to explain why Eli Lilly changed the inclusion criteria for Retatrutide obesity trials to a BMI>35 ONLY. It implies the drug is so powerful, that people with a lower BMI shouldn’t take it, or at minimum should only use lower doses.
Again, that’s unprecedented in medical history.
To finish up the side effects, the discontinuation rate excluding the weight loss stoppages appears at least a bit more reasonable as in most obesity trials the stop rate is usually 5-8% which only leaves the 12mg as an outlier still, but like I said earlier, we need side effects over time and just more data in general to see what’s going on with that dose.
Final thoughts
I’m going to repeat myself; this is unprecedented in medical history. This was the shortest retatrutide obesity trial, and it STILL has essentially matched bariatric surgery outcomes in terms of weight loss. Never have we seen that before. Tirzepatide is a great drug, and it reaches the edge of bariatric surgery, but this profoundly beats what is currently the king of the market. Cherry picking the best tirzepatide trial data showed a 25% weight loss on the highest dose. The 9mg dose beat that in 68 weeks. The 12mg clearly outclassed it. The side effects are an open question and will get answered later. The fact that a good number of patients had to stop due to excessive weight loss is eye-opening as well.
Now what’s even crazier for me to type is that these are NOT the maximum weight loss results. TRIUMPH 1, 2 & 3 are all 80-week trials so they will show EVEN more weight loss which is almost hard to fathom. And not only that but TRIUMPH-1 was extended to 104 weeks for a subset of patients that were still losing weight at week 80! Unprecedented in medical history is going to be a term used OFTEN in the next 6 months as these other trials report out.
Stay tuned, kidney blog incoming next week (I promise)
(As long as there’s no other late breaking GLP-1 news)
References:




