Glucose-dependent insulinotropic polypeptide (GIP) & the heart: Lets drop the pressure and up the beats
Part 1 of this series looked at GIP especially in the pancreas, Part 2 looked at insulin sensitivity, adipocytes and the anti-nausea effects of GIP. And this 3rd post in the GIP series will examine the cardiac and bone benefits of GIP while simultaneously diving deep on the SURPASS cardiovascular outcomes(CVOT) trial results to see if we can see if GIP really is providing extra benefits over just GLP-1 alone. We’ll use multiple prior trials of GLP-1 agonists as our imperfect comparators and see what exactly we can tease out. Let’s dive in!
GIP receptor(GIPR) is found in the heart along vascular smooth muscle cells (VSMCs) and vascular endothelial cells; (VECs) these are the cells & muscles lining our blood vessels. Once again I think a reasonable question to ask is why is a nutrient stimulated hormone receptor found in our heart and blood vessels? Indeed, GLP-1 receptors are also found in these locations as well.

The answer most likely has to do with something we discussed in part 2. Adipocytes. Specifically in a post meal state, increased levels of GIP increases blood flow, especially to adipose tissue. Small studies in healthy human controls who were infused with physiological concentrations of GIP show an increase in blood flow to adipose tissues.
How is it doing this? It is thought to mediate nitric oxide production in VECs. Nitric oxide is a potent vasodilator, relaxing blood vessels which allows for increased blood flow. This has a knock-on effect of lowering systemic vascular resistance which lowers blood pressure. So increased blood flow to adipocytes for triglyceride disposal(see part 2) and a bonus of lowered blood pressure.
In the heart, the GIP receptor seems to increase heart rate, this also goes in concert with all of the above effects, lower vascular resistance and blood pressure causes your heart rate to increase to sustain blood flow. Looking at trial data from the tirzepatide trials this effect is clear, there is a small but sustained increase in heart rate and a sustained decrease in blood pressure usually around 8mmHg systolic, most of which is not weight loss mediated as the effect can be observed in the first 8-12 weeks of dosing. This is also where I have to briefly mention, that GLP-1 as well can decrease blood pressure by increasing urine excretion along with inhibiting central sympathetic tone, and both actions will lower blood pressure as well. With a dual agonist such as tirzepatide, this effect appears synergistic based on trial data.

Pooled blood pressure reductions in SURPASS 1-5 trials with various doses of tirzepatide
In addition to all of the above, GIP agonism also appears to reduce inflammation in blood vessels and limit the proliferation of VSMCs. This is important as excess VSMC growth leads to vascular disease through a process called intimal hyperplasia. This overgrowth of tissue can cause blood vessel stiffness leading to higher blood pressure through the now narrowed vessel, this also slows the flow of blood through the narrowed section increasing the risk of plaque development along the vessel walls further worsening the issue. Delaying or pausing this process with GIP agonism may also cause further reductions in death, cardiovascular disease and its related co-morbid conditions.
With the basic physiology sorted out, I want to review the evidence from the randomized controlled trials we have so far. I’ve included 4 trials that I feel give us the best comparators for what SURPASS-CVOT is reporting out. These trials include SUSTAIN-6, SELECT and FLOW which are all trials with semaglutide. SUSTAIN was for T2 diabetics at high risk of cardiovascular disease, SELECT was for obese non-diabetics with high risk of cardiovascular disease. FLOW was a trial of renal outcomes in diabetics with chronic kidney disease. Finally the REWIND trial is a trial of dulaglutide in diabetics with minimal heart disease and looked more at using a GLP-1 as primary prevention of cardiovascular disease. Eli Lilly is using the REWIND trial as the putative placebo for the SURPASS-CVOT trial so its results will be of most importance.
As that chart below shows, all these trials showed a reduction in MACE. (Major Adverse Cardiovascular Events) Three out of the four trials showed reductions in heart attack and stroke with up to a 40% reduction in the SUSTAIN trial. Only the SELECT (15%) and FLOW (29%) trials showed statistically significant reductions in cardiovascular death. And finally, only the SUSTAIN trial DID NOT show an improvement in all cause mortality.

Statistically significant results are bolded
In fact, the SELECT and FLOW trials showed essentially the same reduction in all-cause mortality despite being two very different participant groups at roughly 20% reduction in mortality. The REWIND trial did show a reduction in all-cause mortality, but it just missed statistical significance(p=0.067) however, it was only the 1.5mg dose and not the maximum 4.5mg dose that is commonly used now. What can we gather from all of this?
In simple terms, mono-agonist GLP-1 medications can reduce incidents of cardiovascular events, (stroke, heart attack) provide some benefit for all-cause mortality depending on the medication, dose and co-morbid conditions, and at least in certain subgroups appears to reduce cardiovascular death as well.
But what happens when you add GIP to the mix with tirzepatide. The short answer is similar to slightly better reductions in cardiovascular events and death, but with frankly astonishing benefits for all-cause mortality and high-risk CKD patients. The long answer will be the rest of this blog.
SURPASS-CVOT was designed to be a trial that compared 1.5mg dulaglutide (GlP-1 mono agonist) vs maximum tolerated dose of tirzepatide over a roughly 4-year period of time. Participants had to be obese diabetics and matched participants as closely as possible in terms of labs, medications used etc. See the images below for more detail:

High levels of statin use, metformin use, ACE/ARB use and surprisingly insulin use

Cholesterol well controlled, however, A1c, and blood pressure were not.
Primary outcomes were cardiovascular in nature, such as MACE, but also included key secondary outcomes such as all-cause mortality, weight loss, change in hemoglobin A1c, change urine albumin-creatinine ratio (UACR), and change in GFR slope and more. As this is a trial with an active comparator, an easy way to remember things going forward is this. If tirzepatide matches dulaglutide we say it is non-inferior, i.e.: similar in a given metric. If it is better than dulaglutide we say it is superior(better) to it.
With that in mind, let’s just dive in, the biggest headline was that tirzepatide was non-inferior to dulaglutide for MACE. This means in simple terms both medications can lower your risk of cardiovascular death, heart attack or stroke, however, that does not tell the whole story here. Look at the graph below:
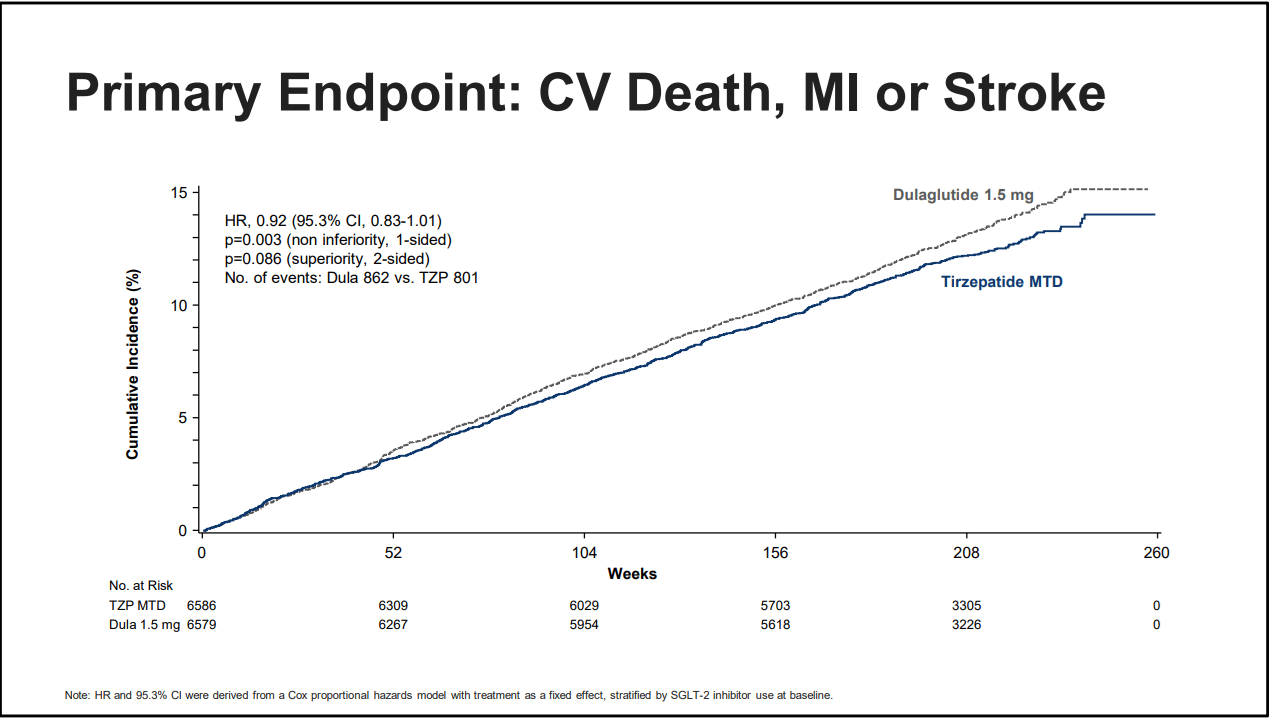
Source: Eli Lilly, EASD presentation 2025
What are you seeing? Do you notice that tirzepatide has a lower rate of events overall, and that the gap is slowly widening as time passes? Did you happen to notice in the far left corner “p=0.086 superiority, 2 sided?” To translate that, it means that tirzepatide is not inferior to dulaglutide at preventing MACE. But look back at the graph. Slowly and inexorably tirzepatide is the better option over time. This will become more clear as we review other data.
But I must dip back into the weeds for a moment to discuss hazard ratios (HR), confidence intervals (CI) and unity. Unity is often just the number 1, or it’ll appear as a dotted line on a graph. Science often uses 1 as a number for both the HR and CI. A confidence interval (CI) is a range of values that is likely to contain the true population parameter. That is, it gives an upper and low range of an effect of something you’re measuring, so if unity is 1, then a CI of 0.74 to 0.94 means there is a 6% to 26% reduction in something. The tighter(smaller the range between numbers) the confidence interval, the more certain we are of an effect. Wide confidence intervals means an uncertain effect, or perhaps too small of a sample size, or other confounding factors. The Hazard Ratio meanwhile is an estimate of risk of event over time. This relates back to CI, a narrow CI means the HR is a more accurate assessment of an event, whereas a wide CI means the opposite. If the CI intervals cross unity(are higher than 1) we often say the event cannot be determined to be statistically significant, that is we cannot say with full confidence that a medication or intervention is more helpful than whatever control we’re testing against.

For more info: tantalusmedical.com/quick-guide-interpreting-forest-plots
Why did I just Reader’s Digest statistics? Because to understand this trial and to be able to compare to other trials we’re going deep on CI and HR. For many of the values we’re about to look at tirzepatide crosses unity, oftentimes just barely which, 1.) yes means it may not be statistically significant but I want to argue it’s clinically significant in the sense 2.) the HR is below 1, and 3.) this is an active comparator trial with NO placebo arm. Therefore it is still providing a benefit and if the HR is below one, that suggests it is probably better than dulaglutide and in some cases, and spoiler alert, such as all-cause-mortality, it is so obviously better that I’ll argue at the end of this blog that tirzepatide should be the drug we should be prescribing for diabetics full-stop.
Now returning to the topic of MACE, we also have a comparison of tirzepatide versus an indirect placebo. We see that there is a 28% reduction in MACE compared to placebo and it’s statistically significant. Looking at cardiovascular death alone we reach the first point where our result crosses unity, but the HR suggests a clinically significant benefit. The HR is 0.75 for tirzepatide but the upper CI is 1.09. There is a chance this effect is not real, especially as only the FLOW Diabetic CKD trial showed similar effects and reduction on cardiovascular death, but it is a tantalizing result nonetheless.

Source: Eli Lilly, EASD presentation 2025
Moving on with this slide we get to the other arrowed section and perhaps one of the most exciting findings from this trial. Against indirect placebo tirzepatide reduced the risk of dying from any cause by 39%. I cannot stress how astonishing this statistic is, none of the other trials of GLP-1 medications have shown such a massive reduction. I want to put this in some context, these patients all had had some cardiovascular event in the past, 80% of these patients were on statin medications, and the average LDL cholesterol was 80mg/dl in these trial patients, meaning their cholesterol was well controlled. What wasn’t controlled was their diabetes and blood pressure.
To be able to have such a massive effect on all-cause death is an astonishing miracle of modern medical science. For further context, the SELECT trial showed a 20% reduction in all-cause mortality and those patients were NOT diabetic. They were arguably a lower risk subgroup and yet, tirzepatide still came out ahead. That is a remarkable feat!

Source: Eli Lilly, EASD presentation 2025 (To quote Neo from “The Matrix;” WHOA)
But where is this change in mortality coming from? Is GIP doing it? Is there something else? I have a couple guesses. First is a combination of weight loss, A1c and blood pressure reduction. Tirzepatide showed greater A1c control, -1.7% vs 0.90%, greater weight loss, -25.2lbs vs -10.2lbs and greater reduction in systolic blood pressure, -6.5mmHg vs -4.2mmHg.

Source: Eli Lilly, EASD presentation 2025

Source: Eli Lilly, EASD presentation 2025
The next reason is renal outcomes and changes in GFR. Kidney disease is the 8th leading cause of death in the USA. Tirzepatide again showed something unexpected and essentially unseen before in medicine. The effect is more noticeable in the high risk chronic kidney disease subgroup so we can start with this graph:

Black line added to show GFR slope difference Source: Eli Lilly, EASD presentation 2025
Two things are immediately apparent, first is that tirzepatide slowed the rate of GFR decline compared to dulaglutide, but what is unprecedented is that the GFR slope actually deflects POSITIVE for the first 2 years of the trial. I’ve even added a straight black line to highlight the effect. To my knowledge, this effect has never been seen in a phase 3 trial for any incretin medication(GLP-1 or GLP-1/GIP agonist) Now there was a drug that was in development called Bardoxolone that showed an increase in GFR but it caused an increase in protein in the urine(albuminuria) and in some patients showed an increased risk of hospitalization. Tirzepatide on the other hand in this same trial showed a reduction in albuminuria.
Tirzepatide significantly reduced albuminuria in both the general CKD population and high risk CKD population by 22% and 26% compared to dulaglutide respectively, though the reduction in the high risk population is confounded by the fact that only 109 tirzepatide patients had data collected at week 260, the effect was greater at week 208 when over 1,000 data points were collected.

Source: Eli Lilly, EASD presentation 2025
This change in GFR slope for the high risk kidney disease population is a huge deal, any delay in progression of CKD will reduce further complications down the road. In the end tirzepatide in this group slowed the rate of GFR decline by over 40% compared to dulaglutide, which is yet another stunning finding. Using the FLOW trial as a rough comparison to another GLP-1 medication, we can again see that tirzepatide is better. Semaglutide showed a GFR slope of −2.19 ml per minute per 1.73 m2 per year over 4 years, meanwhile using the data provided by Lilly we can see a GFR slope of −1.66 ml per minute per 1.73 m2 per year at year 3.
If we extrapolate that out to year 4 it’s even better as the slope in the tirzepatide graph flattens out and using the starting GFR of 52.9 it appears the average GFR at year 4 is 47.5. Doing some quick math shows the GFR slope to be roughly -1.35 ml per minute per 1.72 m2 per year. While I need Lilly to publish the full paper, this is the best guess I can give with the available graph and is almost double the improvement seen with semaglutide which is seen right now as the gold standard for slowing the rate of GFR decline. I’ve made a graph to highlight the differences, including the extrapolated year 4 data for tirzepatide, starting at a presumed GFR of 55 to highlight the differences.

Mind the GFR gap
To wit, this is probably where some of the extra benefit from all cause death is coming from. The risk of all cause mortality was 16.6% on tirzepatide and 19.7% on dulaglutide in the high risk CKD groups. About a 16% difference in death! Add in the other benefits around weight, A1c and blood pressure I believe that is why we’re seeing such improvements.
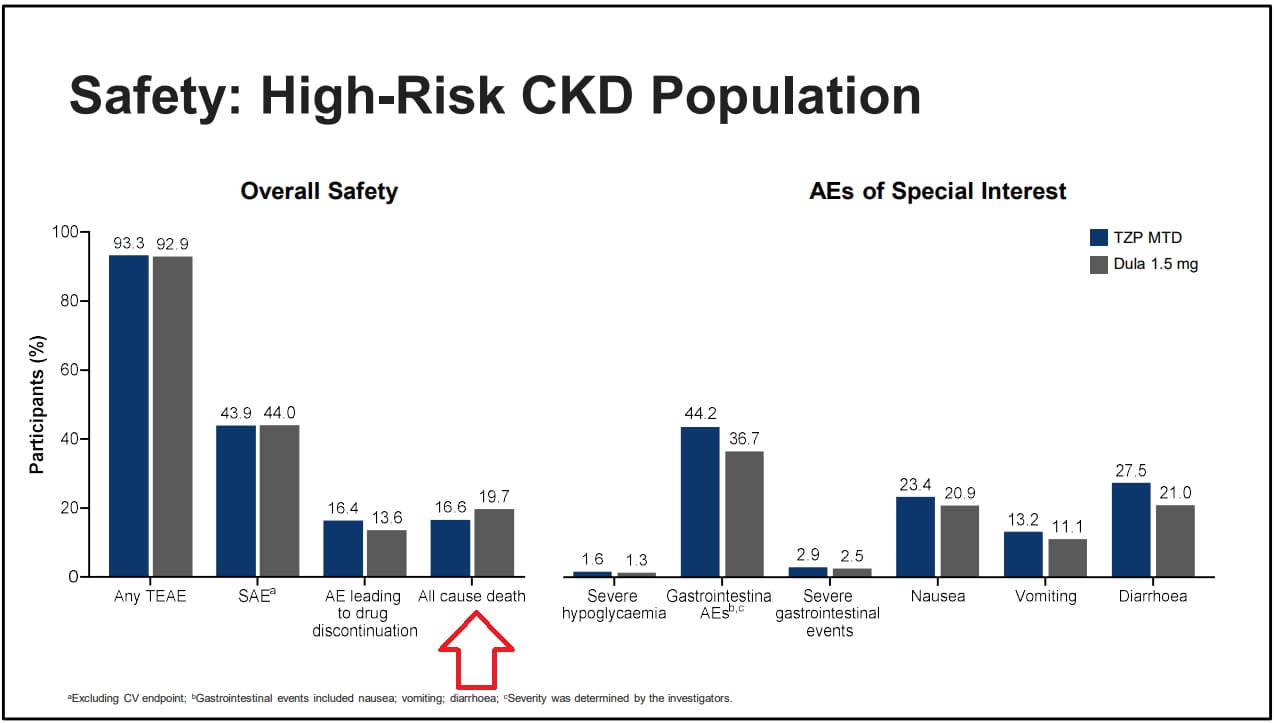
Source: Eli Lilly, EASD presentation 2025
Finally, Lilly has what they consider their primary composite kidney endpoints, this includes persistent macroalbuminuria(>300mg/g), persistent >50% reduction in GFR, end stage kidney disease(dialysis) and death from kidney disease. With this we again see for the overall study population and the high risk CKD population tirzepatide showed a statistically and clinically meaningful benefit for the primary endpoint. In the general population it was especially effective at reducing macroalbuminuria with a 28% decrease compared to dulaglutide. It also preserved GFR better in the high risk CKD group as these patients had about a 27% decreased risk of persistent GFR reduction >50% in this group. While Lilly did not provide numbers compared to a putative placebo, I think it would be safe to say this is a significant renal benefit. I say this again with the CI crossing unity on a couple of these values, but the overall benefits against a placebo should be obvious.

Source: Eli Lilly, EASD presentation 2025, bolded number are statistically significant
Here that same data but graphed out and much easier to visual how tirzepatide is providing a greater benefit for both groups, but especially the high risk group.
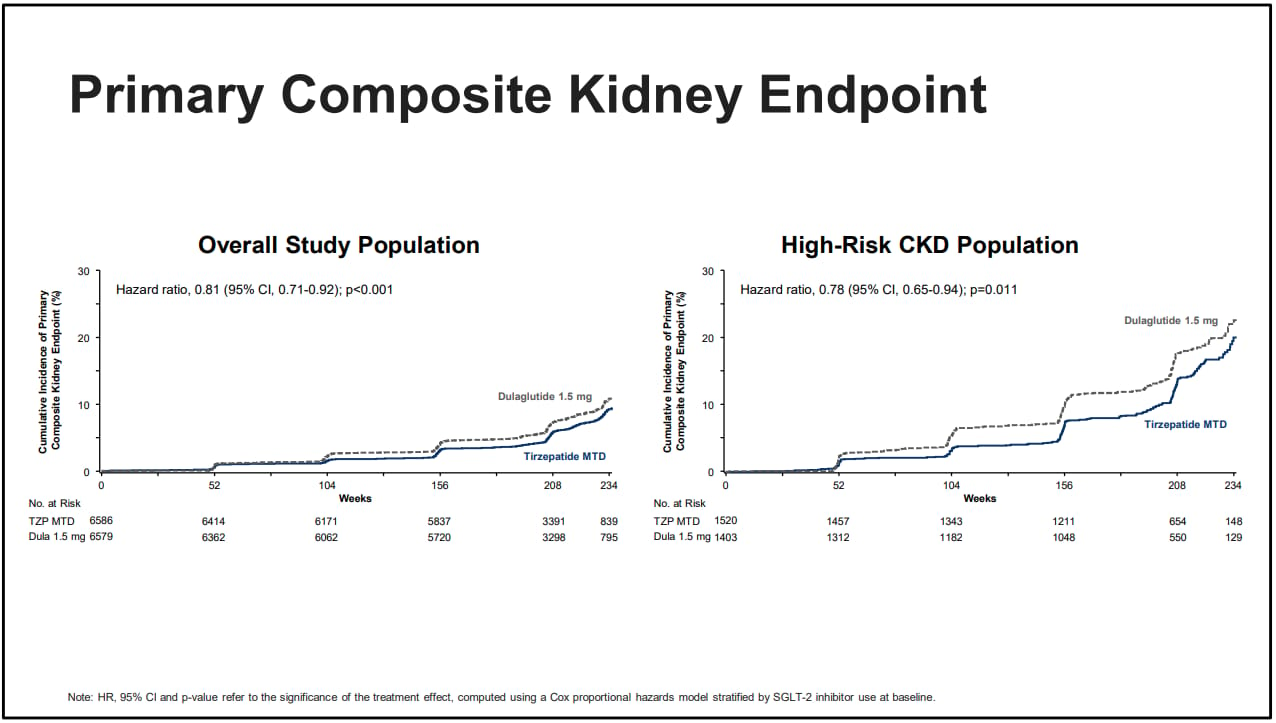
Source: Eli Lilly, EASD presentation 2025, the lines speak for themselves
Using the FLOW trial as an imperfect comparison, the primary kidney outcome in that trial was slightly different as it lacked the persistent macroalbuminuria measure, but otherwise was the same measure. Semaglutide reduced the risk of the primary outcome by 24%. Tirzepatide reduced the risk of primary kidney outcome by 22% versus dulaglutide in the high risk CKD patients and also reduced the risk amongst the general population by 19%. If I can insert my personal opinion here, tirzepatide is most likely better than semaglutide at reducing the risk of renal complications full stop.
For completeness sake, I want to insert two more images, one is the GFR slope for the general population, and the other is the characteristics of the high risk CKD patients so that for the medical professionals reading this, you have an idea of benefit for the general population GFR slope and for just how sick the high risk CKD patients were for this trial.

Again a positive GFR slope for the first year!
The high risk group was decidedly not in a good place. 50% had overt macroalbuminuria, 75% were at least CKD3a or worse, A1c was not well controlled and all had been diabetics for decades.

Source: Eli Lilly, EASD presentation 2025, Nephrologist’s nightmare cohort
That’s where the data runs out until we have the full published data.
I think the point is made that tirzepatide is doing some unprecedented and frankly incredible things. If I may make a couple final points both as a medical professional and as someone who is on a GLP-1 medication. If my diabetic patients aren’t on tirzepatide I am having a discussion with them about either starting it, or switching to it if they’re on dulaglutide or semaglutide. Rare is the trial that shows such compelling evidence of superiority over other medications in the same class. We know tirzepatide is better at weight loss, but now we can point to this data and say it’s better or matches mono-agonist GLP-1 medications across the board. In blunt terms, do you want to lower your chance of dying of anything medically related? Take tirzepatide to treat your diabetes.
As a person taking a GLP-1 it makes me feel personally better about my own long term health seeing these results. I’m certainly not diabetic but it isn’t difficult to extrapolate these data outwards to non-diabetic patients. While we have to wait 2 more years for the SURMOUNT-MMO trial which will examine tirzepatide in the same context as SURPASS-CVOT but in non-diabetics and with a true placebo, I imagine those results will show similar benefits. At the end of the day, faced with my own mortality, I can imagine I will be taking these medications as long as I can. Not only am I at a healthy weight now, but my quality of life has significantly improved and if these medications can help sustain that and reduce my risk of dying, it is worth it to me. The discussion of whether these are short term medications or long term medications is starting to favor long term use in my opinion.
Finally, while these results are truly amazing, we are mere months away from the release of topline phase 3 retatrutide data, and that drug is even MORE effective than tirzepatide for weight reduction, lipids, blood pressure, GFR and more. There is a reasonable chance we might see something even more shocking in terms of health benefits. Stay tuned!
References:








