Glucose-dependent insulinotropic polypeptide (GIP) & insulin, let’s get sensitive
In part one we explored the critical role of GIP in signaling inside the pancreas as paracrine interactions control a large amount of glucose stimulated insulin secretion, that GIP is the dominant incretin in the body and how GIP’s effect is lost in diabetics. I ended with a cliffhanger about GIP’s effect on fat cells and other effects on insulin throughout the body. In part 2 we’ll explore the other effects of GIP, from insulin sensitivity, adipocyte & immune signaling, and finally brain/appetite signaling.
As a primer for what I plan to discuss, it is notable that the GIP receptor (GIPR) is found throughout the human body. GIPR is in adipocytes, brain, lungs, blood/immune cells, bones, gut and obviously the pancreas as discussed last time. This in turn means that GIP has wide ranging effects, for example, the GIPR in the heart seems to increase heart rate when infused via IV into humans while also managing peripheral blood flow and vasodilation, but that is for part 3.
Let’s first explore insulin sensitivity, this in particular may help explain some of the differences in weight loss and A1c reduction compared to a mono-agonist GLP-1 like semaglutide. If I may be frank in my opinion, GLP-1 is a one trick pony in the pancreas. It increases insulin secretion but that’s the majority of its action. In fancy words, it’s an insulin secretagogue, but it doesn’t directly increase insulin sensitivity. GIP on the other hand, DOES significantly increase insulin sensitivity in a mostly weight loss independent manner. We usually measure this with something called Homeostasis Model Assessment for Insulin Resistance or simply, HOMA2-IR.
In a phase 2b study, it was found that 10mg and 15mg tirzepatide increased insulin sensitivity and only 13-20% of that was attributable to weight loss. In the phase 3 SURPASS-2 40-week study tirzepatide reduced HOMA2-IR between 15 and 24% depending on the dose. There was also a reduction in fasting insulin levels of 9-21% depending on the dose in Type 2 diabetics. This trial used semaglutide as a comparator, and HOMA2-IR only dropped 5% and fasting insulin was unchanged, highlighting the difference that GIP agonism was having on insulin secretion.

In the extended 176 weeks SURMOUNT-1 trial graphic above, we can see in non-diabetics as well, a large reduction in insulin secretion suggesting increased insulin sensitivity and more efficient uptake and clearance of glucose during a 2-hour oral glucose tolerance test. Unfortunately, HOMA-IR numbers were not calculated for this trial, but the authors did include that fasted insulin levels dropped 54% when pooling the 3 tirzepatide doses together.
But how is it doing this?
What if it’s all just futile?
Enter part 2 the adipocyte. GIP binds to the GIPR in adipose tissue and activates the expression and secretion of an enzyme called lipoprotein lipase (LPL) in the presence of insulin after a meal. This helps to clear triglycerides from the blood by transporting them to subcutaneous adipocytes for storage. In doing this GIP agonism helps to slowly reduce the stores of fat around visceral organs, which plays a large role in systemic insulin resistance.
GIP agonism has another effect, when insulin is low in between meals, it increases the production of adiponectin from adipocytes. Adiponectin is a hormone with multiple effects that include increasing insulin sensitivity, breaking down lipids, increased glucose uptake into cells and decreased gluconeogenesis in the liver. In plain terms, this breaks down fat cells releasing triglycerides back into the blood, and upregulates fatty acid oxidation, thereby burning those triglycerides as energy.

Tirzepatide significantly increased fat oxidation in humans
If this seems futile to you, first storing the triglycerides, then releasing them again to be burnt for fuel, then storing them again and repeating this over and over again, you’d be correct. We even have a name for this in science: futile cycling.
In fact, we even have some clinical evidence of this, in the graphic above in a small phase 1 trial, a small but statistically significant amount of extra fat was oxidized daily and participants spent on average an extra 4 hours per day burning fat as measured by indirect calorimetry.
With insulin sensitivity and adipocyte signaling handled and how these two mechanisms help with the action of GIP agonism and how both play a role in weight & A1c reductions, we can move onto immune modulation and brain/appetite signaling.

Futile cycle of lipids via GIP agonism
Keep calm and immune on
GIP and GLP-1 both play key roles in inflammation, immunity and signaling in the brain, so you may notice some overlap in this section, in fact GLP-1 deserves its own newsletter about its role in these functions, but that will come at a later day. For now let’s start with GIP and immunity.
GIPR is found in hematopoietic stem cells, these are the cells that are the precursor to essentially our entire immune system, red blood cells and platelet production. From there we see the GIPR is preserved mostly in myeloid cell lines, including monocytes, macrophages, but also is included in some T cells as well. But why is it there?

The answer seems to be modulating immune responses. Now a big caveat is that all of this evidence is in preclinical models, so results must be taken with a grain of salt as to whether all of this translates to human biology. With that being said GIP seems to decrease gut inflammation and by extension systemic inflammation. Specifically, a lack of GIP receptors on immune cells seems to increase the expression of calprotectin in white adipose tissues in mice leading to an increase in metabolic and inflammatory disorder. Furthermore, the loss of the GIP receptor on immune cells leads to a reduction in anti-inflammatory cytokines. As such one potential purpose of GIP on immune cells is to tamp down the inflammatory effects produced by large amounts of adipocytes, especially in obese states.

The upshot of this is a synergistic reduction in inflammation as GLP-1 also reduces inflammation via its own mechanisms. This could in part be part of the reason many patients on tirzepatide subjectively report less pain, swelling and feelings of inflammation. It could also be a reason that Eli Lilly is studying tirzepatide in inflammatory bowel disease, as calprotectin is used as a biomarker for ulcerative colitis.
No nausea, no problem
Our final topic is reviewing how GIP signals in the brain in terms of appetite, nausea, vomiting, neuroprotection and ‘assisting’ GLP-1 in signaling in the brain. In the central nervous system, GIPR is found in the cerebral cortex, hippocampus, and olfactory bulb. Importantly for our discussion, GIPR is also expressed in multiple locations in the hypothalamus that play a critical role in regulating energy homeostasis, appetite and aversive behaviors.
Moreover the hypothalamus, area postrema, and median eminence at the base of the hypothalamus are what we call the circumventricular organs of the brain. These sections of the brain do not have the tight blood brain barrier that encompasses most of the rest of the brain. Instead the capillaries here allow small molecules, hormones and other substances to pass through, this allows direct sensing and signaling including GIP and GLP-1. Considering the hypothalamus is critical for appetite and energy expenditure this makes some sense from an evolutionary standpoint to allow both hormones to signal the brain.
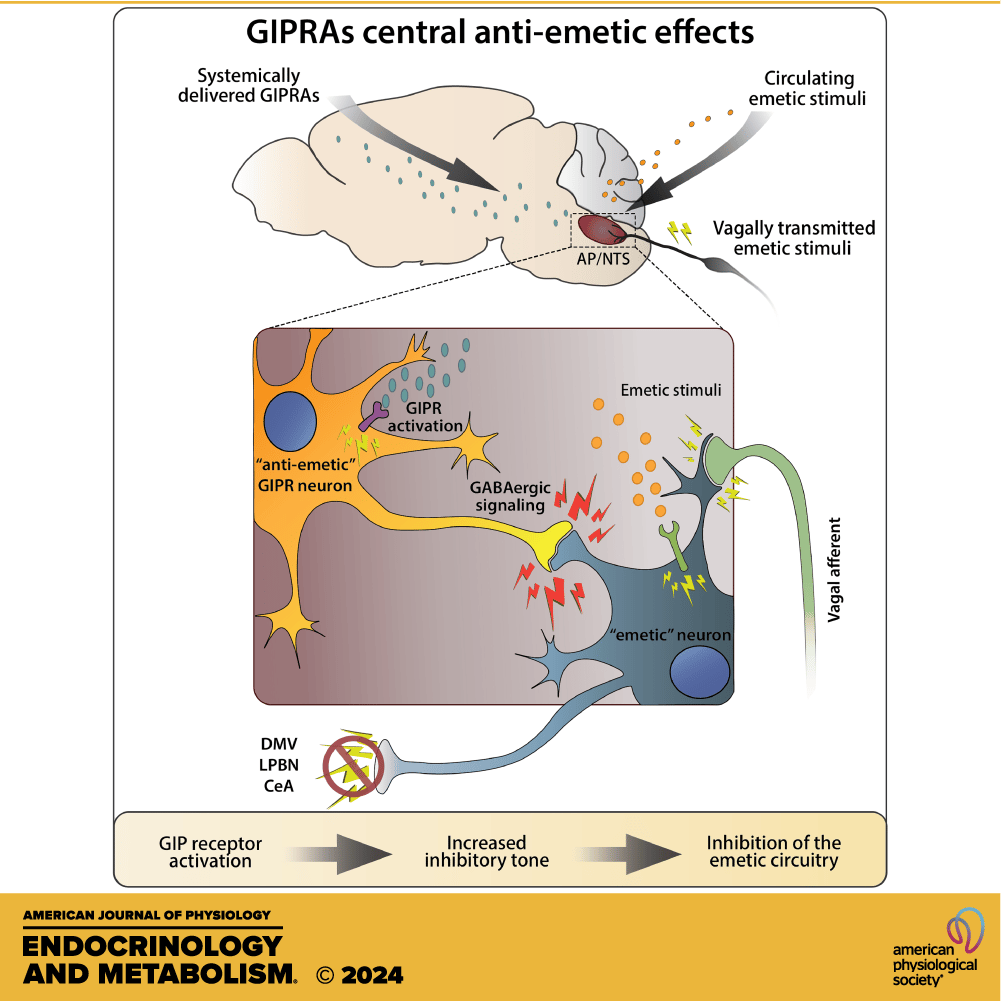
The first and easier topic to discuss is GIP and reductions in nausea and vomiting. As mentioned above we have a part of our brain called the area postrema, in this area of the brain are neurons that when activated signal the brain to have an aversive response to stimuli. In plain terms, it makes you nauseated and more likely to vomit. In patients receiving chemotherapy, this area of the brain typically is stimulated and is why so many chemotherapeutics cause nausea and vomiting. GLP-1 does the same thing, with its signaling from vagal afferent nerves from the GI tract converging here and causing an emetic stimulus.
However, emerging evidence in both human and pre-clinical trials shows that GIP receptors exist in this area and signaling through GABAergic pathways, actually reduces the vomiting signal. In an elegant trial in 2024, researchers at Eli Lilly were able to show that the addition of a long-acting GIPR agonist significantly reduced the total number of GI side effects from liraglutide treatment by 16% and reduced nausea by 15% in a small pilot study. This may explain why, when comparing tirzepatide to semaglutide trials, we generally see a lower rate of side effects for tirzepatide.
Don’t forget your appetite
Moving onto appetite regulation and other effects. As I am not a trained neurobiologist, I will do my best to keep this simple, but neuronal circuitry is a complicated and ever evolving field, partially thanks to these medications. Now caveat is most of these studies are in mice, because dissecting living human brains is generally and broadly frowned upon.
The first is a very recent discovery that GIPR in specific cells called oligodendrocytes in the median eminence seem to be responsible for the increased weight loss efficacy of dual GIP/GLP-1 agonists. GIP signaling here seems to increase the production of new oligodendrocytes, which increases brain access of GLP-1 through vascular plasticity to signal arginine vasopressin (AVP) neurons. Whew. That sentence is a mouthful.

In more plain terms, GIP signals the growth of new cells, this allows more blood flow through the median eminence which allows GLP-1 to signal a specific set of neurons, which leads to additive weight loss. Diagram below if you’re more of a visual learner.
The proof of this is that when the research group blocked or deleted the GIPR from this area of the brain, the weight loss matched that of a mono-agonist GLP-1 medication, suggesting that GIP is helping in a synergistic manner.
Finally, the last effects that have been discovered in rodent models is that GIP seems to have a modest effect on appetite and body weight signaling through the hypothalamus. And perhaps, most compelling, but unproven benefit at this time in humans, is an increase in neuroplasticity, increase in memory and decrease in brain inflammation. If found to translate to humans, could be an extremely important finding in relation to age related memory loss and other related issues such as dementia.


Various neurological actions of incretin medications
I’ll pause there as this post is already heavy on science. Part 3 will review the bone and heart health implications of GIP agonism, but this time we’ll be reviewing it in the context of the SURPASS-CVOT trial which is a 3.5-year study on Mounjaro (tirzepatide) and how it reduces the occurrence of cardiovascular disease and death in Type 2 diabetics. Stay tuned!
References:












