Glucose-dependent insulinotropic polypeptide (GIP), another forgotten and misunderstood hormone
This article will be part science, part history lesson. GIP, like glucagon is a hormone that was discovered long ago, and like glucagon, was essentially forgotten about by most as a target for drug development simply because it was thought it didn’t work in diabetics.
Let’s start our story in December 2018 in the journal Molecular Metabolism when Eli Lilly published this paper titled: LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept - ScienceDirect At the time there was some mild interest in this trial but this was two years after the seminal clinical trial showed semaglutide reduced the risk of nonfatal heart attack and stroke, and a year removed from the other initial trials of semaglutide showing profound benefits for diabetics in terms of A1c and glucose control along with some weight loss over trials that lasted from 30 to 104 weeks. Everyone was excited for semaglutide. GLP-1 was starting its meteoric rise to social media fame.
But those with a keen eye would have noticed something very important even back then. This molecule in Type 2 diabetics with its cryptic alpha-numeric name nearly matched the weight loss seen from semaglutide trials in only TWENTY-NINE DAYS. Moreover, the reduction in glucose seen in those 29 days would average out to an A1c reduction that was also equal to or better than semaglutide. Perhaps more tantalizing at the time was that in healthy, non-diabetic patients the weight loss over 10 pounds in 29 days. At the time that was unprecedented level of weight loss, but the focus in 2018 was still on diabetes.

A preview of what GIP could do in only 29 days
We now call this molecule tirzepatide, you might know it as Mounjaro or Zepbound. Fast forward to the summer of 2025 and it has rapidly overtaken Ozempic/Wegovy to become the most prescribed GLP-1 medication in the USA. But how did we get here, and why is GIP so important? For that we have to go back to the 1960s and the initial discovery of GIP.
The search for incretins
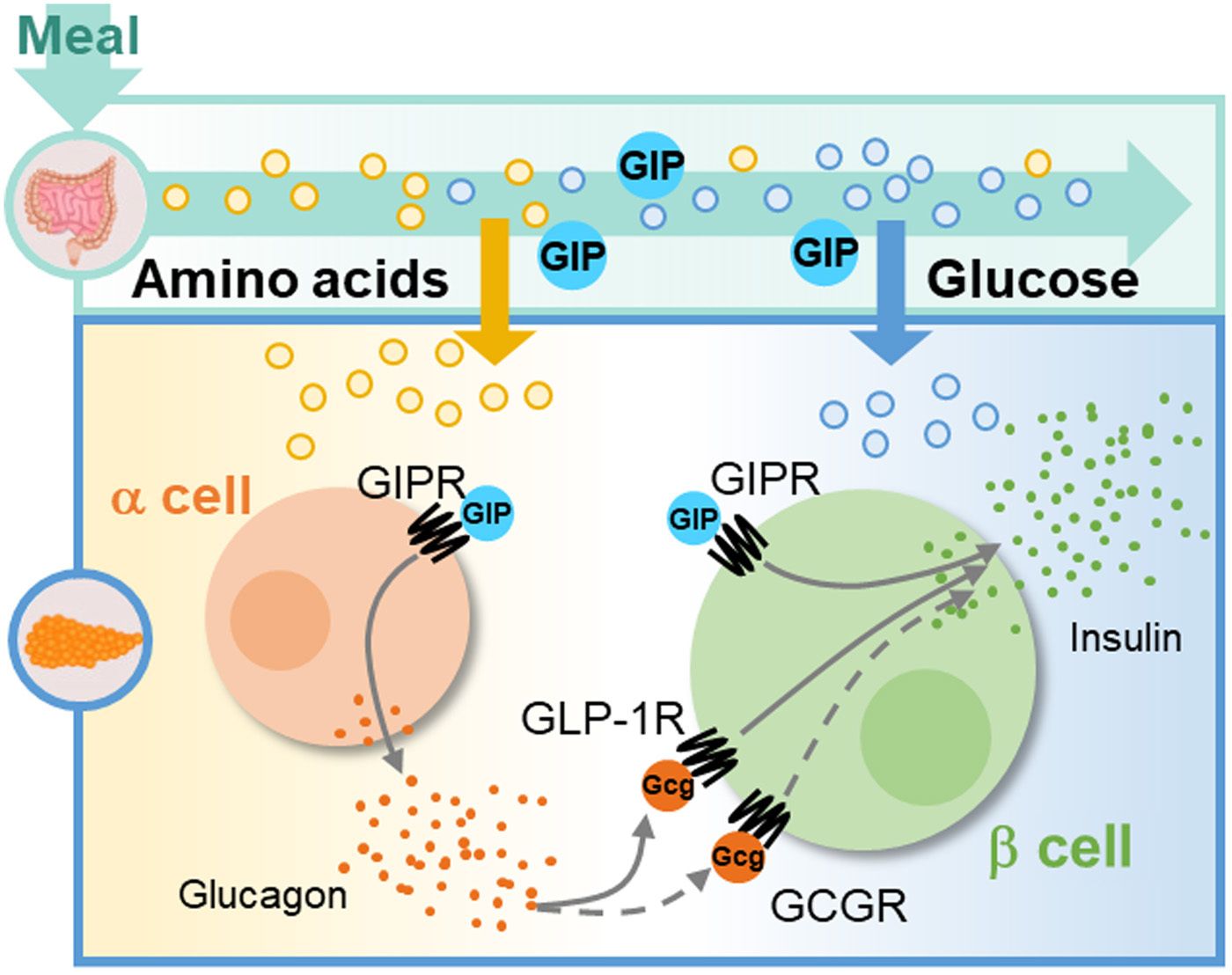
Before I go any further on our history lesson, let’s just breakdown the name of GIP, because for once, scientists gave us a name that directly relates to what it does in our body. Insulinotropic in lay terms simply means insulin stimulating or causing the release of insulin. Glucose-dependent is exactly what it sounds like, to activate the insulinotropic effect, GIP depends on your body’s current glucose level for activity. If your glucose is not at a certain level then the effect of GIP may be decreased or non-existent, and that will be important for our history lesson. My goal of this explainer is to show that GIP is THE most important physiological incretin in the pancreas, controlling the action of both alpha & beta cells. I will review both its pancreatic effects in this article and then explore the other benefits in the next part of this series. I also hope to show that GIP, and NOT just glucagon, plays a contributing role to the development of T2DM.
Let’s start with some basic biology, in our pancreas we have islets, which are a collection of cells, which contain multiple cell types, but critical to this topic are the alpha cell and beta cell. In simple terms, alpha cells secrete glucagon and beta cells secrete insulin and this will be crucial to telling the story of GIP. Our pancreas also contains other cells, such as delta cells, but for today, that role is not important.
In brief, GIP was discovered in the 1960s long before GLP-1. Two different groups of scientists were experimenting with intestinal mucosal extracts to find compounds that lowered blood glucose besides glucagon. One group was experimenting with dogs and when this extract was given to dogs this first group noticed it had two effects, it inhibited gastric motility and reduced gastric acid secretion in the substance. They eventually purified and isolated a substance they called Gastric Inhibitory Polypeptide (GIP). The other group of scientists were using similar extracts and gave this extract as a co-infusion with glucose to humans which then accelerated glucose lowering and was accompanied by increased insulin levels. However, the extract did not have an effect on glucose tolerance or insulin levels in Type 1 diabetic patients suggesting that the extract harbored a hormone with insulinotropic actions.
These two groups then combined together and infused purified GIP and glucose in human subjects which confirmed that it helped lower glucose while increasing insulin secretion. This confirmed GIP as the second discovered incretin hormone (Glucagon is an incretin and it was discovered first but not fully recognized as an incretin until much later) Further experiments showed that GIP acts directly on the pancreas for its insulinotropic effects. In the early 1980s, Werner Creutzfelt’s science group showed that neutralization of GIP in the gut of rats only diminished the incretin effect by about 50% suggesting there was another incretin hormone at work, but that’s the story of GLP-1 and not our focus. Further studies of GIP alone showed it did not slow gastric motility and did not reduce gastric acid secretion; the name was therefore changed to, glucose dependent insulinotropic polypeptide.
GIP is primarily produced in K cells of the upper intestine, but GIP receptors (GIPr) are expressed throughout the body, including bone, adipocytes, stomach, central nervous system, and of course, the pancreas. The focus of this article will be primarily on the pancreas. The expression and function of GIP receptors in other tissues and organs warrants an entire article separate from this discussion.
Where does GIP come from?
GIP is secreted from K-cells in the intestine. Its secretion is stimulated in various manners and has been well described in the literature. Generally fasting GIP levels are <20 pmol/L and around 300 pmol/L an hour after a meal with a half-life of 5-7 minutes in the body. Just like GLP-1 is degraded by the DPP-4 enzyme. GIP secretion increases with higher calorie meals compared to lower calorie meals and can be augmented(increased) by a small amount of protein ingestion prior to the rest of the meal. On a macronutrient level, GIP is stimulated by sugars, fats, and specific amino acids, more on this in a bit.
The incretin effect and GIP
50-75% of the insulin response to oral glucose intake is mediated by the incretin effect and is most effective in lean individuals but suppressed in patients with high BMI and/or oral glucose intolerance. The incretin effect is further blunted in patients with Type 2 diabetes, usually accounting for 35% or less of the insulin response in these patients. Essentially GIP is unable to enhance GLP-1 secretion in patients with Type 2 diabetes UNLESS you give them insulin or GLP-1 for at least a few weeks before hand, this then seems to ‘resensitize’ the body’s beta cells to actions of GIP.

With that in mind, studies on humans and rodents, show that GIP is actually the dominant incretin hormone, as it has an ability to act on both the alpha and beta cell, and that when a GIP antagonist is given, insulin secretion is blunted more than if a GLP-1 antagonist is given. In summation, the decreased incretin effect of GIP seen in T2DM is thought to be from impaired beta cell function along with downregulation of the GIP receptor on the beta cell.
Controlling Alpha and Beta Cells
For decades, the literature around type 2 diabetes has centered on insulin resistance, and inappropriately high levels of glucagon as two of the causative factors for overt development of type 2 diabetes. But what if, GIP also had a role to play in the development of diabetes? Perhaps dysregulated or downregulated GIP signaling in the pancreas is also part of the milieu of diabetes development? Let’s review what GIP does in a healthy lean pancreas first and perhaps find our answer at the end.
Studies in healthy humans show that GIP infusion will increase glucagon levels after an overnight fast and that GIP will increase glucagon secretion in a reciprocal manner during normoglycemia and hypoglycemia, but not under hyperglycemic conditions. In plain language, GIP can help stimulate glucagon secretion to raise blood sugar when it is low or keep it stable, but true to its insulinotropic nature will not stimulate glucagon when your blood sugar is high. But again, this is in healthy patients.
However, in patients with Type 2 diabetes, GIP DOES stimulate glucagon secretion, and the insulinotropic effect of GIP is ALSO blunted in these individuals. This leads to an interesting hypothesis, is the GIP actually the problem instead of glucagon? Further studies in diabetics show that an infusion of GIP while eating a mixed meal actually further increased glucagon levels and post meal blood glucose levels.
But wait, in biology, things are rarely so black and white. Instead, if we refer back to my article on glucagon agonism, the keen reader may remember that glucagon is insulinotropic and acts on the beta cells to stimulate insulin secretion through paracrine mediated crosstalk between alpha and beta cells. I propose my own hypothesis; this increased glucagon secretion is simply the body trying to compensate in any way possible to lower blood glucose. But in Type 2 diabetes everything is working against your pancreas, increased peripheral insulin resistance, decreased beta cell insulin secretion, fatty liver disease, a disrupted liver-alpha cell axis and more are simply worsening the problem, and we enter a vicious feedback loop. All this to say, GIP signaling is probably contributing to the pathogenesis of type 2 diabetes. This would also explain why when scientists first discovered GIP, it didn’t seem to do anything for diabetics when infused alone.
The next logical question then becomes, why then is GIP agonism seemingly so useful for treating diabetes?
The answer comes in multiple parts. First off, let’s circle back to my first paragraph of what happens with GIP in healthy individuals. Researchers had a hypothesis on how to get GIP to work again. They would take diabetic patients, corrected their blood glucose first with other medications over about 2 weeks and then infused GIP. In these diabetic patients, GIP started to work like it should, no increased glucagon secretion, but signaling for more insulin secretion during times of hyperglycemia.
The second and related part of this answer is that GIP is the predominant incretin in the body. As seen in the graphic shown earlier, GIP drives essentially double the nutrient derived insulin secretion that GLP-1 does. This goes back to the fact that GIP can signal both the alpha cell to secrete glucagon which then signals the beta cell to stimulate insulin release and GIP alone stimulates insulin secretion from the beta cell directly.
If you’re a different kind of learner, here it is broken down into a chart showing what’s happening:

This chart also allows me to bring up the final point of why GIP is the superhero incretin. It is also stimulated by amino acids. In experiments if you infuse GIP, you’ll get a little bit of glucagon release, and similarly if you infuse just amino acids, especially certain ones like alanine, you’ll get some glucagon release but infuse both at the same time and you’ll get a massive and synergistic release of glucagon from the alpha cell. This suggests that GIP is the primary conductor for glucose homeostasis as it can respond to nutrient stimulation from all 3 macronutrients, but especially for protein and carbs. This can be seen in the graph below. Graphic A shows just alanine stimulated glucagon release, graphic B shows GIP stimulated glucagon release, and columns C and D show what happens when you add the two together, the effect is relatively massive with over 7 times more glucagon release!
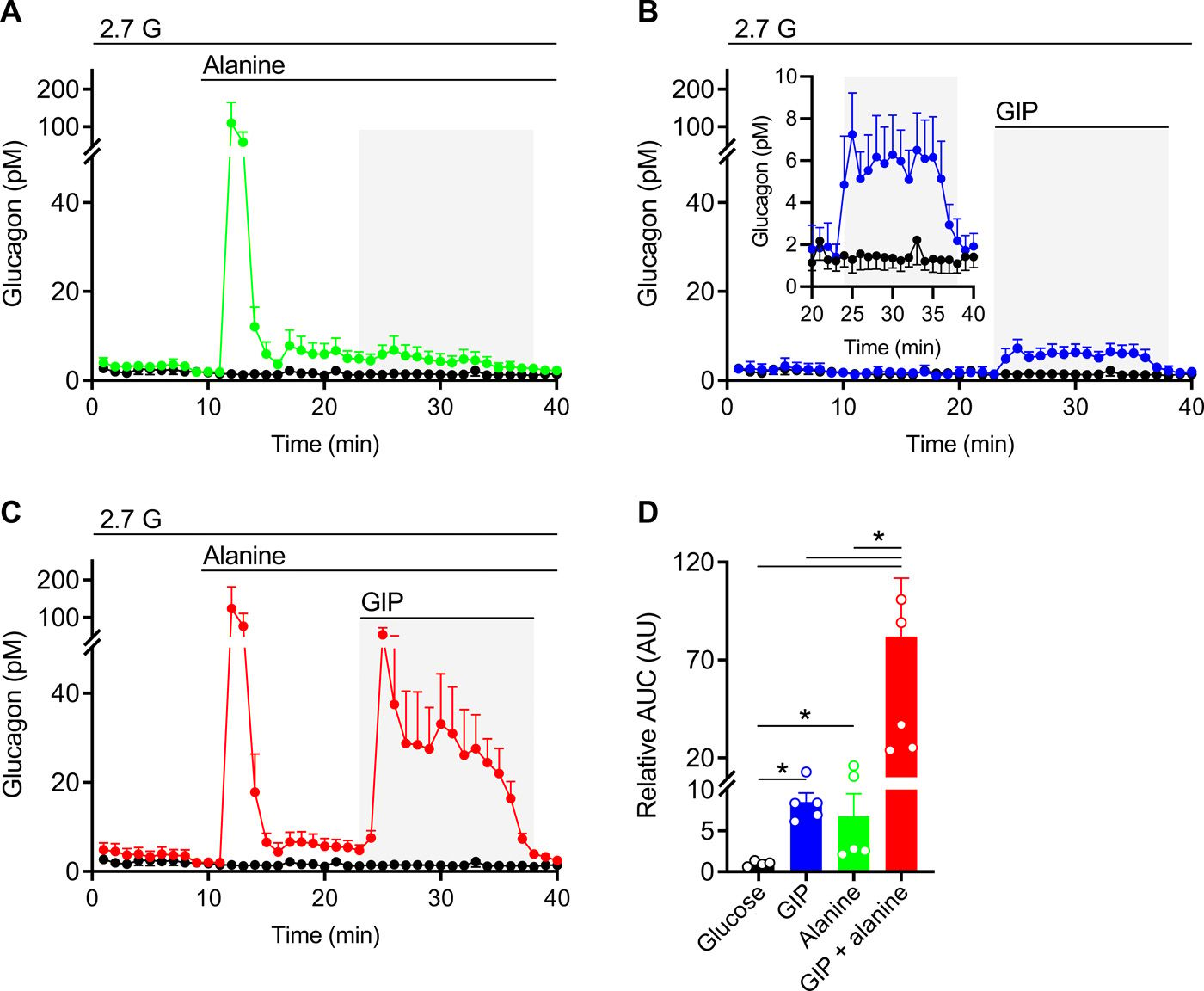
Given this, it is now understandable and perhaps easier to see that GIP dysfunction in Type 2 diabetics breaks the incretin effect. Not only are you losing the primary driver of insulin secretion, but it’s now abnormally signaling for glucagon as well.
With that in mind, let’s circle back to why GIP/GLP-1 dual agonists will probably become the de facto standard of treatment for Type 2 diabetics in the future, and leave our story on a cliff hanger for next time when we review the other actions of GIP. With a dual agonist, in a diabetic, initially the GIP part will not contribute much, at least at first, but as the GLP-1 portion of the drug reduces blood sugar, the GIP part will ‘turn on.’ At that point, suddenly everything happens. Look again at the tirzepatide graph from earlier, specifically part A and E. One is body weight, and the other is blood glucose graphed over 7 points in time. Notice how it takes about 2 weeks for the body weight and glucose lowering to really kick in? At day 15 weight loss suddenly accelerates, and by day 22 the glucose level is almost back to normoglycemic without big post-meal spikes? That’s probably GIP signaling correcting itself.
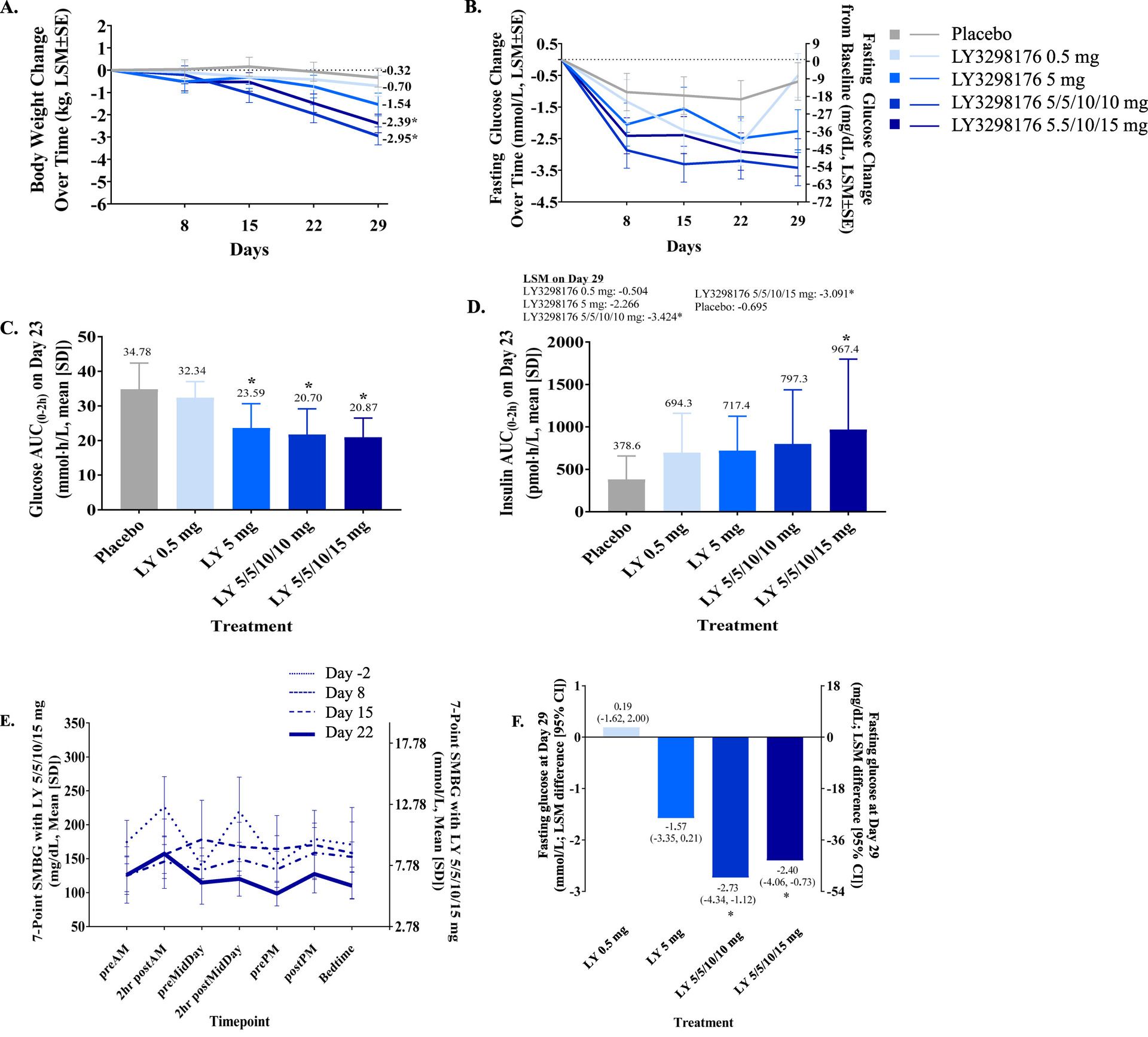
But there’s also something more happening that is helping with glucose disposal and weight loss. Because incretin drugs are exogenous, and long lasting, we’re pressing on that GIP receptor for a really long time, far longer than what our body can do naturally. With that, funny things start happening in fat and adipocyte cells in our body, and it has some interesting effects on insulin as well, but that is a story for next time. Stay tuned!
If you’re feeling particularly punchy for a much more technical & scientific discussion of this topic, Dr Jonathan Campbell, recorded this lecture in 2022 which was the inspiration for this article:
References: