I fibbed again, my GLP-1 and the kidney post is coming soon, but it’s a labor of love at nearly 5000 words, and for now these results were compelling enough that I felt it needed a quick post!
This blog has already written twice about orforglipron, the oral non-peptide GLP-1 medication being developed by Eli Lilly and now on track for FDA approval in 2026. As one could imagine, given the record sales and incredible demand for GLP-1 medications worldwide, they aren’t the only ones developing products.
Enter today’s latest drug, aleniglipron by Structure Therapeutics. It is yet another entry in the Tortured Pharmaceuticals Vowels Department(with apologies to Taylor Swift) Funny name aside, these results have been hotly awaited for some time now by those of us in the know(including your author) This is two phase 2b trials called ACCESS. One with ‘standard’ dosing and the other testing higher doses to see if there is any difference in weight loss or side effects.
Let’s talk molecule shape first, aleniglipron is what is known in the pharma spaces as a “me too drug.” That is generally understood as targeting the same receptor as a known drug for similar effects, in this case targeting the GLP-1 receptor for treatment of obesity and diabetes. More classic examples would include cholesterol medications(statins), and acid reflux medications (proton pump inhibitors including the notorious examples of omeprazole & esomeprazole back in the 90s and early 00s.)
But this “me too drug” is different from orforglipron in a couple ways. First it’s been modified just enough to avoid Eli LIlly’s patent attorneys, I’ve included images that show the changes in the molecule:

The same!

But different!
Then secondly, and I’m not a biochemist but apparently these changes shorten the half-life compared to orforglipron, reduce the side effects (more on that in a minute) and lower the potency, so the doses we are about to discuss are much larger than the 6mg/12mg/36mg that Eli Lilly tested in phase 3.
Here’s the trial design and then the demographics with the starting BMI highlighted by me:


Notice the BMI is 39 which is quite high! Lilly recently did the same thing with Eloralintide, by recruiting at a higher BMI you have more runway for patients to lose weight, which in my opinion gives a better idea of what the drug is capable of. The downside is that patients need to lose more weight to reach the usual 5%, 10% etc markers that we use to assess efficacy.
What did Structure find? A drug that at least from phase 2b, appears to be better than orforglipron in almost every way and a company that while very small, is attempting to be Eli Lilly in how nimble it is with regards to the trials of this drug. As a friend of mine put it, they're waving a sign to Big Pharma companies that says, “PLEASE BUY US OUT.”
In raw numbers these match orforglipron for weight loss but in a much faster timeframe and without a plateau yet at 36 weeks and also with a dose increase at week 36, continued to lose weight for at least another 8 weeks as shown in this slide:


It isn’t significantly more weight, and I suspect weight loss would plateau around week 52 again in line with other mono-agonist GLP-1 medications, but it is proof of continued loss.
Also, kudos to Structure for allowing the placebo patients to start the actual drug after 36 weeks. That’s a nice incentive to stay on trial!
Here’s the weight loss broken down by percent lost, along with a note that aleniglipron reduced blood pressure in line with other GLP-1 agonists:

Next is the high dose ACCESS data, these included tested doses up to 240mg(!!) over 6 times higher than orforglipron max dose! These results are frankly the most impressive to me, this is following a trajectory that slightly exceeds the weight loss expected with 2.4mg of injectable semaglutide for weight loss with a LARGE caveat that this is a trial of only 61 patients:

But if you’ve read my posts before you’ll know this is where I hold up a hand and say, now for the bad news. Side effects.
This appears to be poor tolerability but with a peak behind the curtain, maybe not!
First off, the side effects as a chart:

Oof, that nausea rate seems really really high, doesn’t it? On par with the infamous MariTide data. Same with the vomiting rate. Super high!
But look at it broken down over time:
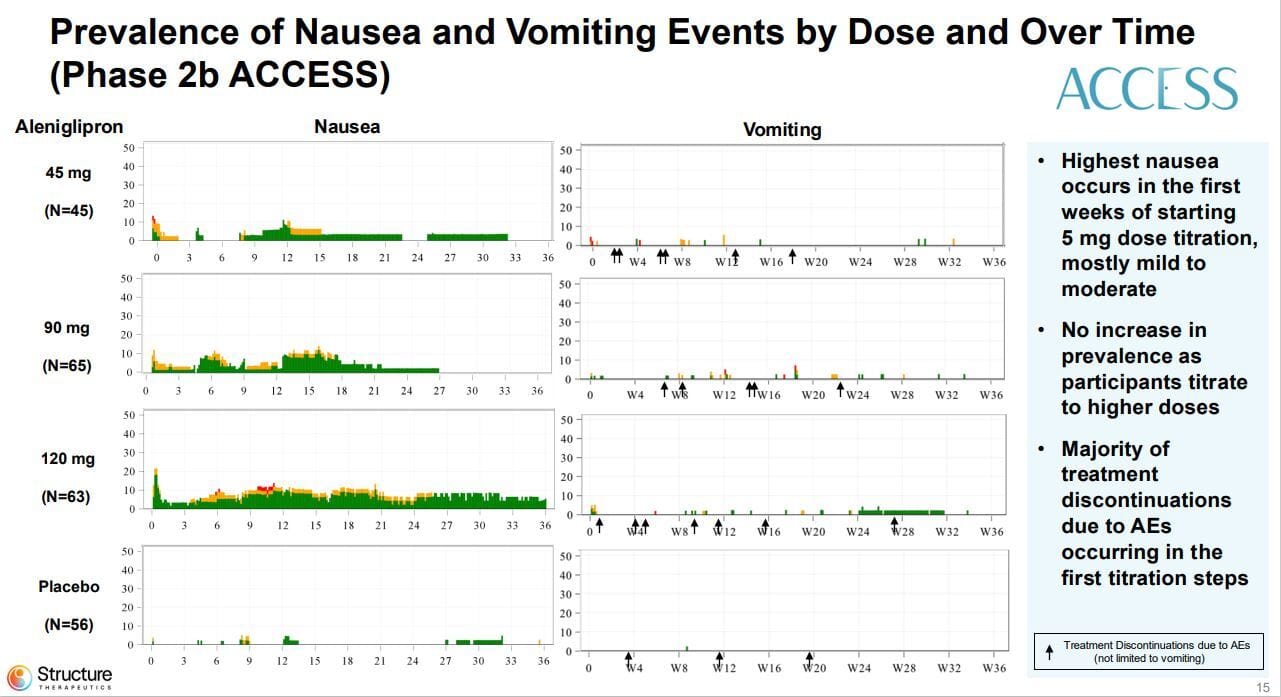
Green-Mild Yellow-Moderate Red-Severe
Maritide, this ain’t, especially the 45mg and 90mg doses which show good tolerance after about 6 months, in line with the injectables.
Large spike to start, as expected but it mostly evens out and starts to get better the longer patients are on the drug. You’ll also note, vomiting is never very high and those little arrows under the vomiting graphics are when patients dropped out, mostly they’re clustered in the first 12-16 during dose titration. This suggests either slower titration or lower starting doses might help. They also didn’t report anti-emetic use, which may also help get patients through the first few months (and frankly is more realistic to how we dose patients in the real world, Zofran is undefeated) So while as a raw percentage, it appears bad, the actual reality over time suggests perhaps the nausea is more intermittent between patients which is why the absolute rate is so high.
In what is also a first, because I usually complain about dose titration being too rapid, Structure Therapeutics figured that out and is ALREADY testing starting out at a 2.5mg starting dose and after 10 weeks, initial results are promising, with a noticeable drop in both nausea and vomiting by percentage as seen here:

That’s significantly better!
This is something I’ve come to expect from Eli Lilly in terms of being nimble with changing protocols on the fly. Structure essentially confirmed that phase 3 would start with 2.5mg as the starting dose and I think that makes sense in terms of tolerability, but will it change weight loss? That’s an open question.
Finally, Structure presented liver safety data because there is a very valid concern that these unusual looking molecules may cause liver toxicity after Pfizer had a failed oral GLP-1 non-peptide drug that caused liver injury.

As you can see the answer is yes, there were some elevations in liver enzymes. However, Structure claims all cases of liver enzyme elevation resolved on their own without needing to stop treatment. That’s good, but Lilly reported similar events with orforglipron so it’s still an area to watch going forward.
To wrap this up, this is an exciting day for oral options but with a few important caveats. The liver enzyme elevations will need to stay low in phase 3 for any hope of approval. I also would have appreciated it if Structure had shown AST/ALT levels over time for the aleniglipron groups. If AST/ALT was going down for the vast majority of patients over 36 weeks that would make me feel better about this drug. Next is side effects, but I think the 2.5mg starting dose answers that question. Phase 3 trials need to happen for approval but Structure barely has the cash for that, so who’s buying them out? This is clearly a promising drug in a massively underserved market but they’ll need lots of help to go this alone, so ultimately I think someone buys them out to bring this drug to market. Finally, will it matter in the end? Even if phase 3 trials started by the summer of 2026, this drug isn’t seeing FDA approval until 2028 or 2029. By then, the market may not have room anymore, even for something best in class.
References:

