After reviewing new molecules for the ADA 2025 conference, I’d be remiss to not review the new data and molecules of interest for Obesity Week 2025, especially since I actually went to the conference! Obesity Week isn’t as high profile as some of the other conferences, but it still is a place for new data to be presented. And this year there were 5 in particular I’d like to highlight. One is an old friend, orforglipron, but new trial data this time. The other 4 are new, Eloralintide, HRS9531, Mazdutide, and MET-097.
I want to start with orforglipron as its familiar at this point in time. When last I discussed it was the Achieve-1 trial which was a short trial in diabetics. This time it’s two trials, ATTAIN-1 and 2. Both were 72 week trials in overweight and obese patients with ATTAIN-1 being non-diabetics and ATTAIN-2 being in diabetics.
This means we get a much longer, much more detailed look at the effects of this drug along with side effects and more. So let’s dive in and see what we find
ATTAINing modesty is great sometimes
One aspect of the obesity medicine space is the race for faster & more weight loss, more receptors, more of everything. It is somewhat refreshing to see a GLP-1 mono-agonist that if I can think of one word to describe I would say it’s modest weight and modest side effects but great at the extra benefits.
What is also interesting is that the weight loss in the diabetic trial and non-diabetic trial of orforglipron were nearly identical at the top dose! That is very unusual in the GLP-1 space, look at the weight loss graphs below:
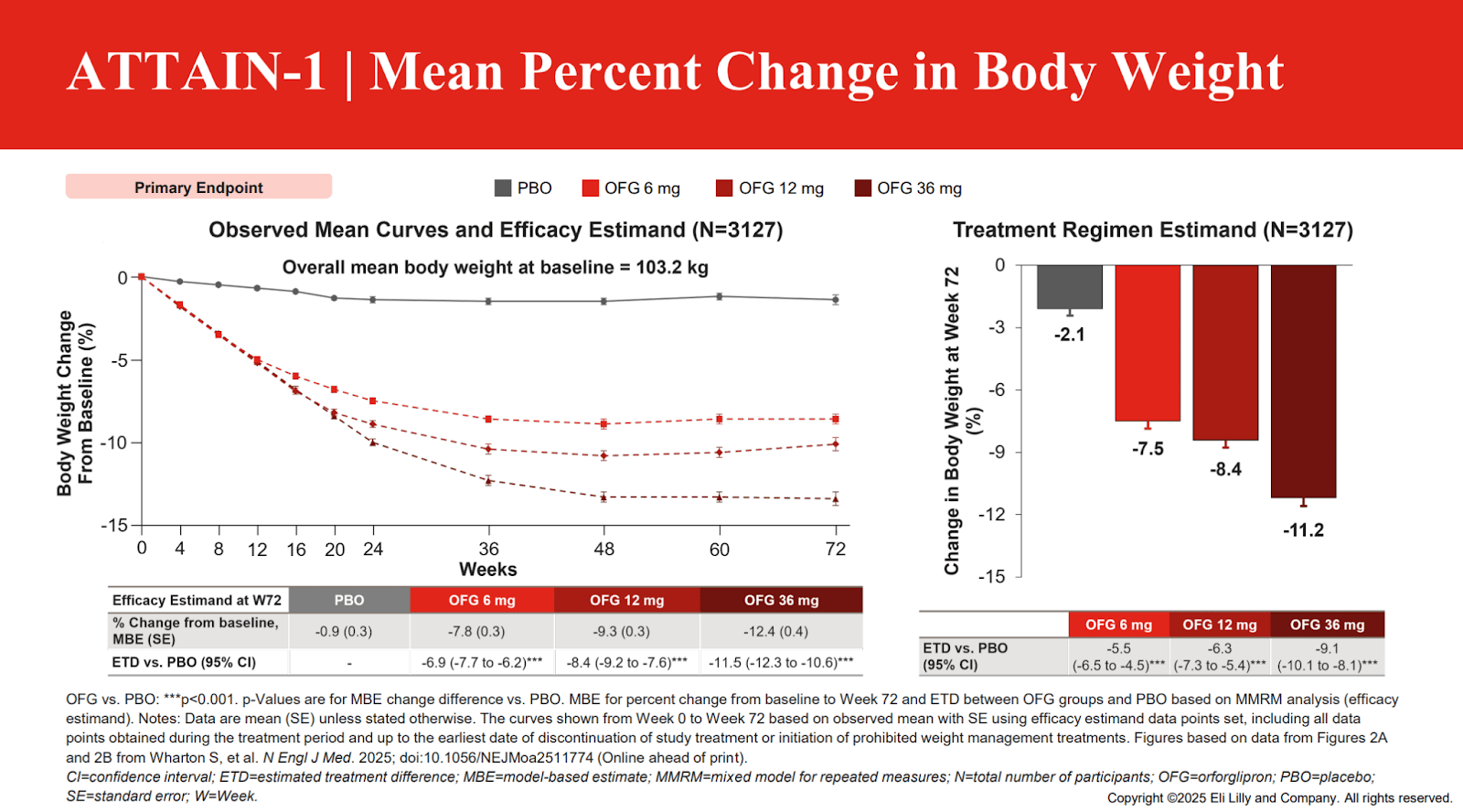
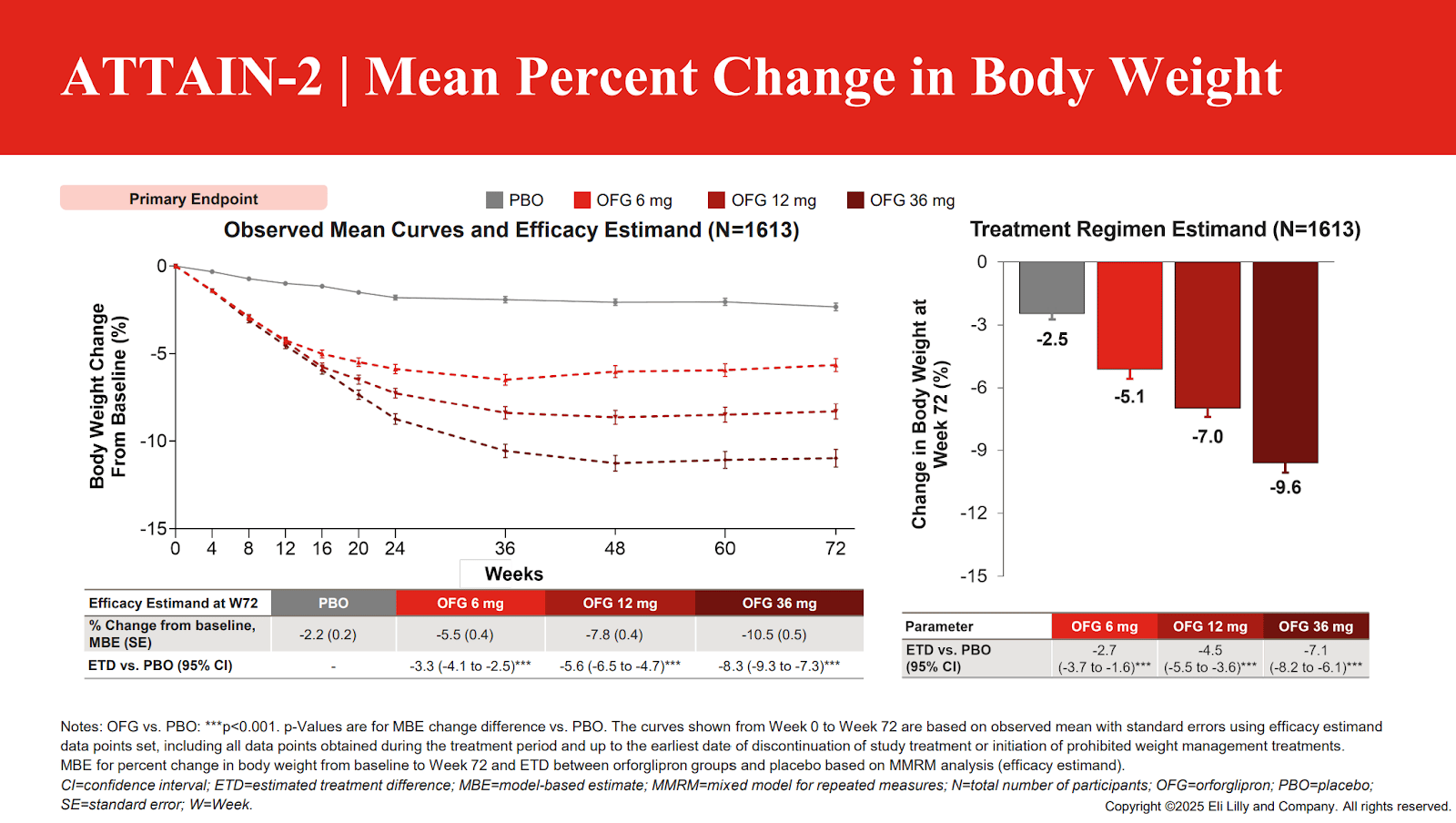
Only about a 1.5% difference in effect. This is curious and strange overall for multiple reasons. The first is that Eli Lilly was expecting greater non-diabetic weight loss with orforglipron based on phase 2 trials, and that apparently never happened for reasons that are still unclear. Expectations from phase 2 were pointing towards 14-15% weight loss vs 11.2%, but this is why we perform clinical trials! On the other hand, the diabetic weight loss was perhaps better than expected.
Moving on from there and trying to keep this as compact as possible, in ATTAIN-2 there were significant reductions in fasting glucose and A1c as expected for a trial of diabetics. Efficacy is on par with injectable semaglutide with up to 1.7% reduction on the highest dose, and change in fasting glucose from -30 to -42mg/dl which is a significant improvement in glycemia.
Next up we’ll tackle side effects which is in my opinion one of the rate limiters for my personal excitement for this drug class. As we can see in the images below side effects tended to be a little higher than injectable medications and I have a hypothesis as to why (more on that in a minute.)
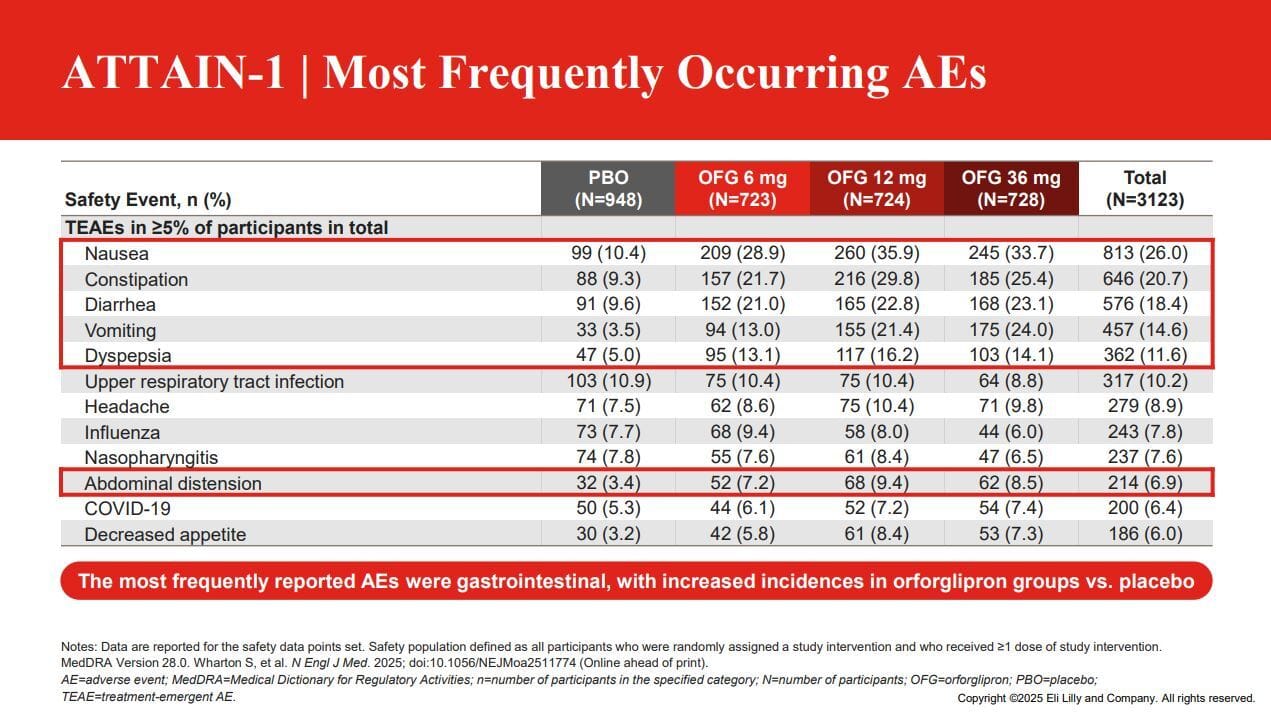

But regardless of whether you look at ATTAIN 1 or 2, the rate of diarrhea, constipation, nausea and vomiting were all similar. I personally don’t love that the odds of these happening are basically 1 in 4 patients, but at the same time over time the rate does decrease with time. But can I get patients to take it long enough for that to matter? Time will tell.
So why is the rate of side effects higher?? It has to actually do with pharmacokinetics. I mentioned in my first discussion of orforglipron (Link here: ADA Conference 2025: Orforglipron & Bimagrumab) that it requires no special dosing as it is a traditional small molecule drug. It can be taken with food or drink without issue. Compare this to oral semaglutide which has to be taken on an empty stomach to work, and any food intake will worsen drug uptake. Well Lilly was kind enough to share this slide about how long a dose of orforglipron lasts.

As you can see there is a spike in blood level that occurs around 6 to 9 hours after a dose. The estimated half-life is 48 hours. As noted in the slide bioavailability was high at 79% but there is a small reduction and delay in absorption when taking the medication with food. Time to reach steady state blood level would be about 11-12 days if I’m mathing correctly and stay with me here, half the month is spent just reaching a new steady state and then you’ll get dose titrated up again and have to start all over again, this is probably driving some of the side effects. You can even see the dose increase spikes on the side effects over time graph. Combine that with people being told they can take the pill whenever means one day they might take it with food, another day on an empty stomach and that can and probably does lead to wild swings in drugs level in the blood, and if research has shown anything consistent with GLP-1 agonists it is that having big spikes and swings in drug levels tend to lead to increased side effects.
My personal hypothesis is that contrary to what Lilly is saying with “dose anytime with no special instructions,” I’d bet a fair sum of money that taking orforglipron with a snack at the same time each day would lead to dramatically better tolerability. To be even more specific, I imagine taking it at night with a snack before bed would probably be the best-case scenario as you’ll be asleep with the drug level peaks and therefore are in the drug trough for the daytime hours. It also begs a question of, would orforglipron be viable as a drug if dosed every other day? Would this reduce side effects? Whether Lilly would actually run trials like this is unknown, and I’m just a random internet person, but you can bet I’ll be suggesting nighttime dosing with a snack to my patients who want to try this drug.
Whew that was long winded of me, let’s wrap this up with blood pressure reductions and quick thoughts. Lilly has announced that orforglipron would be put through clinical trials to be used as a medication for HIGH BLOOD PRESSURE and that absolutely caught my eye. When they flashed this slide I immediately understood why.

If you had essentially normal blood pressure orforglipron does not do much of anything for your blood pressure. But if you HAVE high blood pressure, the reductions are stark and large, even on the lower dose. This essentially mirrors results seen with retatrutide which also caused extremely large drops in blood pressure in hypertensive patients with a much more muted effect in normotensive patients. Digging through literature this effect was noted in Tirzepatide and Semaglutide as well. What is happening there? I’m not sure, but my next topic is GLP-1 medications and the kidney. My best guess is some sort of activation of the RAAS system is happening to lower blood pressure along with the increase in urination seen with GLP-1 medications.
With all this in mind, I am gently excited for orforglipron. Cardiometabolic benefits are similar to injectable medications with decreases in lipids, blood pressure, hsCRP, and glucose. Weight loss may or may not be as great, but for a patient with a BMI of 30 and hypertension who hates needles, this could be a great option.
What if Tirzepatide and Semaglutide made a baby?
One of the big trends in all of pharmaceutical development is that China has absolutely exploded onto the scene with multiple start-ups and larger companies developing various drugs across a spectrum of diseases. The GLP-1 space is no exception. HRS9531 is being co-developed in China by Hengrui Pharmaceuticals and by Kailera Therapeutics in the United States. It is a GLP-1/GIP agonist and while my subtitle may seem cheeky, it’s actually true. This peptide has the same potency as semaglutide at the GLP-1 receptor, but only about half the potency at GIP as tirzepatide.
Tirzepatide is reversed, more potent GIP, less potent GLP-1 so HRS9531 is basically a mirror universe Tirzepatide. If you’ve read my series on GIP, you may guess what this means for patient outcomes. If you guessed similar weight loss with more side effects you’d be exactly correct!
This trial is a phase 3 trial but importantly it was performed in China, it was not a global trial and with that there are caveats! With that being said the trial was 54.5% female, was relatively young, 34 years old, and had an average BMI of 37. Weight loss over 48 weeks results is listed in the graphic below:
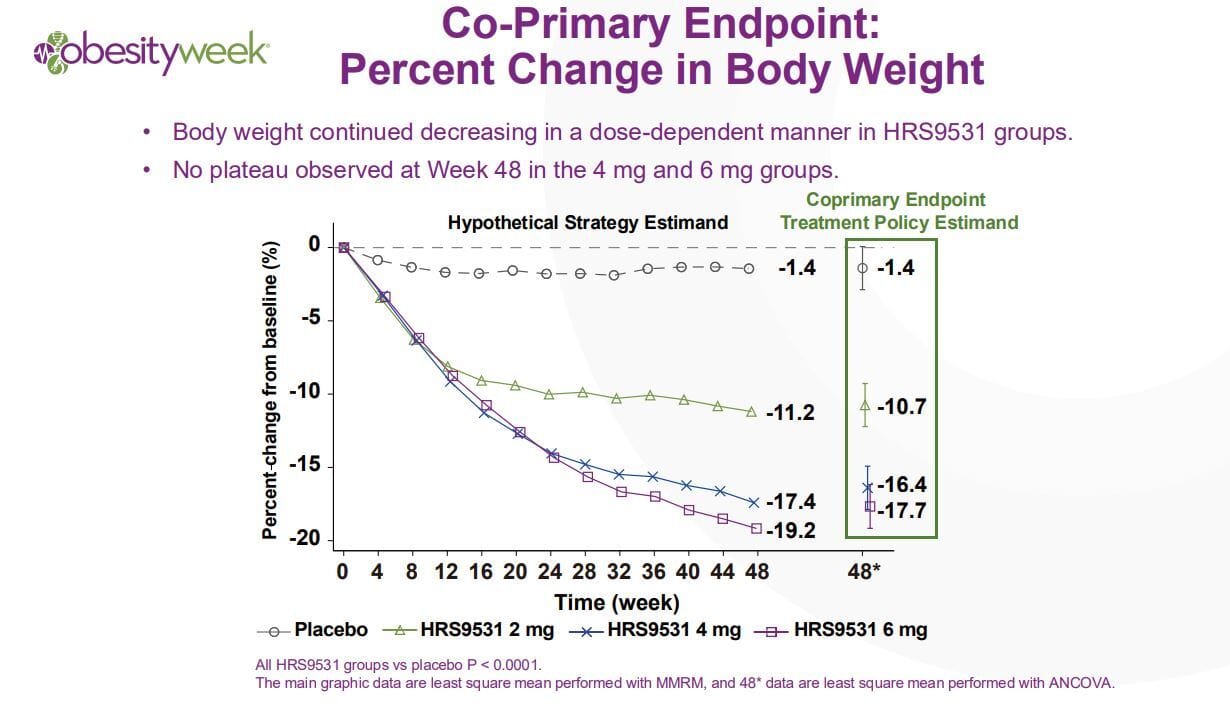
Along with breakdown by weight percentage lost:
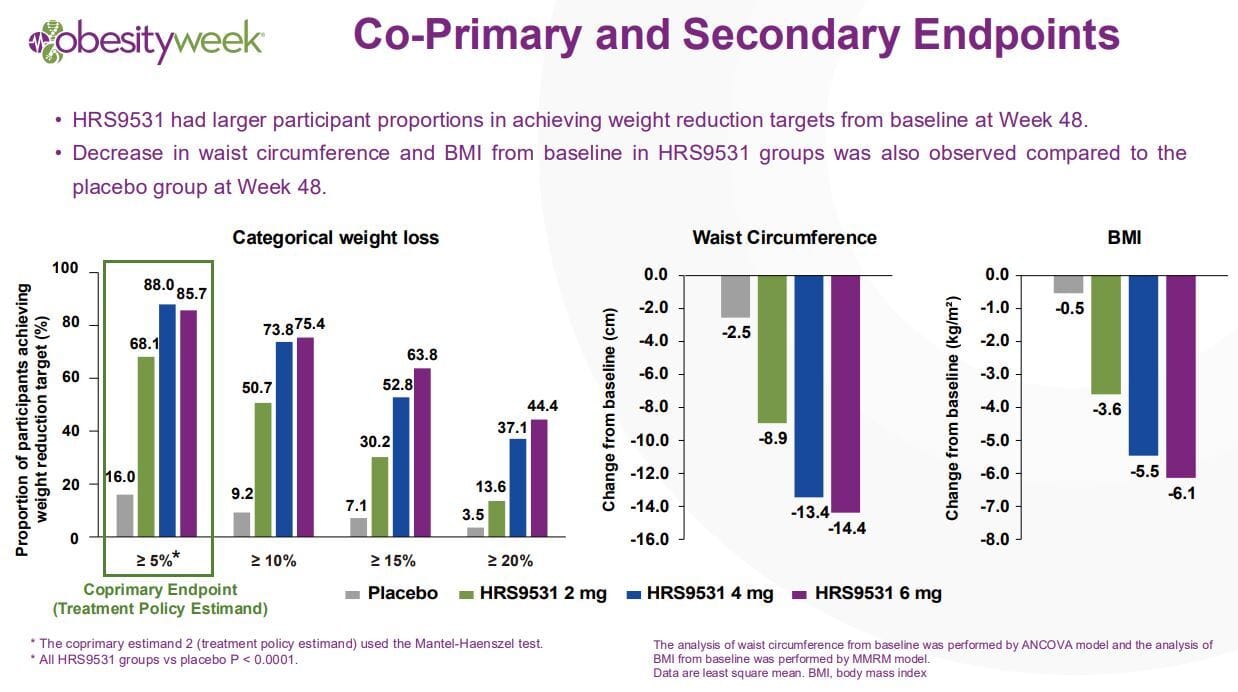
Here’s the cardiometabolic benefits

Well, that’s quite impressive don’t you think? And it essentially is a mirror image of the weight loss and metabolic benefits seen with tirzepatide at 48 weeks. That settles it then right? It’ll be the next big hit? No, allow me to pump the brakes because just like my ADA writeups, we are again in side effect land.

Highlights are mine
The side effects for HRS9531 are essentially in line with orforglipron and in some respects, especially vomiting and diarrhea, are worse. It has less nausea than tirzepatide, but again the vomiting and diarrhea are noticeably worse. And for some strange reason they did not list out constipation rate which is a very curious omission. And to be perfectly fair to Kailera, I think the 4mg dose is probably the best dose in terms of blending weight loss with manageable side effects.
All this to say, these are competitive results however the rates of diarrhea and vomiting could be a problem if they continue to develop the 6mg dose, and for Kailera, they need to conduct trials in the United States and elsewhere. As of time of publication, all further trials were happening in China with no phase 2 or 3 trials active anywhere else. I have a sneaking suspicion that Kailera will end up being bought up by a much larger pharmaceutical company for global development. It is a promising molecule, but we need a global trial if this has any hope for approval in the United States.
MET-097 What if semaglutide lasted a month?
What if I told you that you could dose a GLP-1 med once a month and it would allow for weight loss like semaglutide but with perhaps marginally better side effects? Would you be willing to try something like that? In the summer I offered critique to MariTide which is also a once monthly injection, but one with significant side effects the first few weeks. Metsera took a slightly different approach, but like everyone else in the GLP-1 space found out that actually slow titration is better. This theme will repeat again with eloralintide. It’s the big pharma equivalent of trying to make “fetch” happen. It’ll never happen. Just stop.

Anyways, onto the data AND drama around Metsera. First, they’re developing multiple GLP-1 & Amylin medications. Second is that Pfizer and Novo Nordisk engaged in a bidding war for the company with Pfizer eventually winning out at a price of $10 billion. So this drug, MET-097, going forward will be managed by Pfizer. So what did Albert Bourla buy himself?
He bought a GLP-1 monoagonist that has some different properties. It has a uniquely long half life of about 18 days. This is beneficial in multiple ways, namely, monthly dosing after initial titration dosing, even more prolonged receptor activation along with less peaks and troughs in drug levels, which are thought to contribute to side effects.
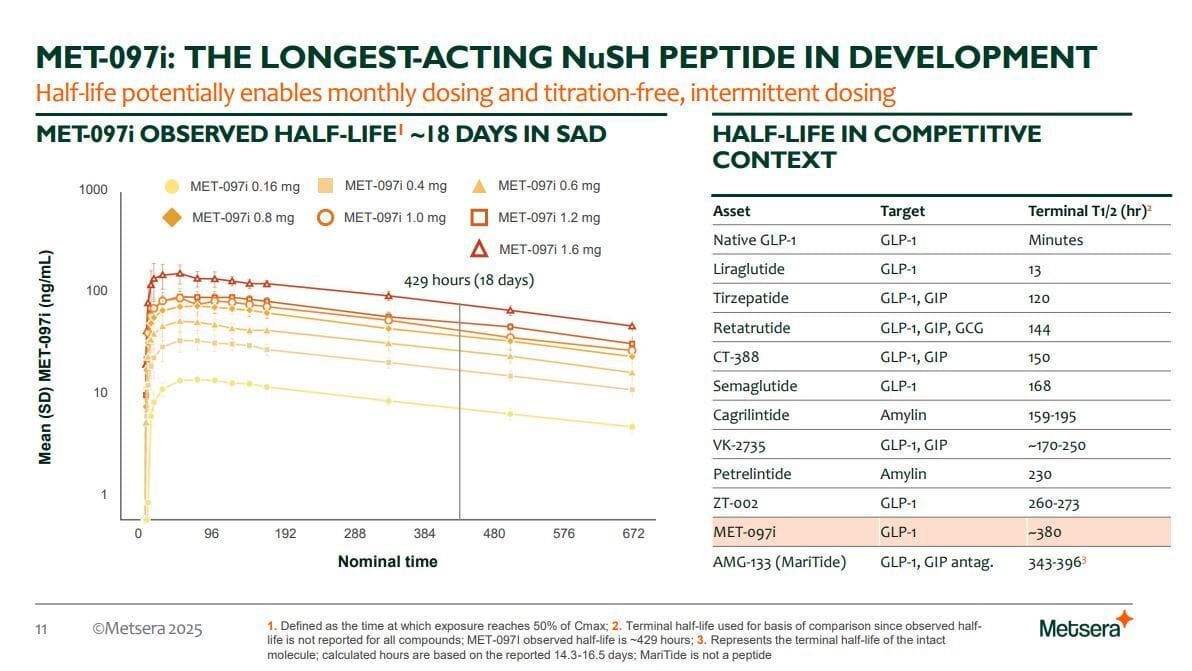
But how much weight loss and how are the side effects? I know I was harsh in my opening paragraph about not doing dose titration but the drug does work! And it causes significant weight loss at 36 weeks across 3 different doses.

Metsera claims that MET-097 has ‘dual agonist-like weight loss’ at 36 weeks, I want to pump the brakes on that right now. The highest dose 1.2mg does match the weight loss curve of 5mg of tirzepatide, with around 14% loss but certainly not the higher doses of tirzepatide which were around 17-18% at 36 weeks. That being said, this at minimum should match and possibly exceed the standard 2.4mg dose of semaglutide for weight loss. Now Metsera claims lower side effects! Is that true?

About the same as the drugs, we’ve looked at so far. Despite this new strategy of ‘ultra long’ acting GLP-1 agonist, the side effect profile is basically the same as almost every other drug in the class. And once again, they tried to make fetch happen without titration, but then backtracked to show that, well, actually, titration reduces nausea, vomiting and diarrhea. Also, like Kailera, they excluded constipation for some unknown reason.

Titration! It works!
With that I’ll note this final slide showing the plan for phase 2b with monthly titration, then going to a once monthly dosing scheme. Stay tuned in the coming years to see what becomes of Pfizer’s newest GLP-1 medication.

Eloralintide, finally breaking the GLP-1 weight monopoly?
I have to admit a bias. I was not sold on the idea of using Amylin agonism as a weight loss medicine. Not that I didn't think it could work! Novo showed that it can with cagrilintide. But rather I didn't see anything that set it apart, the weight loss was marginal, there were no new cardiometabolic benefits, and in the trials of CagriSema it seems to synergistically worsen side effects. So imagine my cognitive dissonance getting shocked when Eli Lilly not only showed that Amylin can play in the sandbox with the big multi-agonists, but that it can do so with perhaps more tolerable side effects. And most importantly Eli Lilly did what I thought was a great job explaining the WHY. But also they tried, like Metsera and Viking Therapeutics and others to make ‘fetch’ happen. It didn’t happen. Phase 3 will include a monthly titration schedule just like GLP-1 meds.
So first let’s look at what & why on Amylin.
Amylin is co-secreted with insulin at a ratio of 100:1, that is for every 100 molecules of insulin, 1 molecule of amylin is secreted. Like GLP-1, amylin is thought to slow gastric emptying, reduce glucagon secretion, increase satiety and reduce appetite, and to be frank, I personally didn’t do a deeper dive on Amylin because when Novo Nordisk presented CagriSema at the ADA conference they were sparse on the details beyond talking up synergy with GLP-1.
Lilly gave us the why in much better detail. Let’s start with the fact that when building an amylin agonist, one must also consider calcitonin. As these two slides show, amylin acts on 3 amylin receptors, AMY1, 2 and 3. Calcitonin can act on both amylin and calcitonin receptors. Furthermore these receptors have “RAMPS” or receptor activity modifying proteins, RAMP1 & AMY1 act on fat storage/adiposity, RAMP3 & AMY3 seem to act on eating preference and glucose control. AMY2 is thought to play a role in digestion of starch/carbs. The calcitonin receptor(CTR) was considered by Lilly to be not worth targeting, it has functions around calcium regulation and bone growth, and the literature is mixed on its effect on weight loss. It also seems to have potentially considerable effects around mood and anxiety. More on that later.


With all that written, Lilly decided to target AMY1 specifically. It has 12 fold higher potency for AMY1 over CTR and 11 fold higher potency for AMY1 over AMY3. It is still a full agonist for all 3 receptors but it is selective for AMY1 so it is more likely to bind to this receptor while having more muted effects on the other two. As this next slide shows, this was very deliberate. They selected the AMY1 receptors because they presume it will have less side effects. The SCT receptor seems to drive many of the side effects seen in other non-selective amylin agonists cough cagrilintide cough The shade in this slide is not subtle.

In plain terms, Lilly posits that selectively targeting AMY1 reduces food intake & body weight with less taste avoidance (aka food still tastes good!) with less GI side effects that seem to be driven by the non-selective activity of cagrilintide on all these receptors.
Finally, they presented this slide showing that Amylin seems to act on two brain regions, the area postrema (AP) and the arcuate nucleus (ARC) and that with damage to the AP you lose essentially all of the actions of amylin.

I’ll point out that there are many question marks on this slide as we know much less about amylin than we do GLP-1. Regardless, amylin without question reduces food intake and in other studies, binge/hedonic eating behaviors. With all that intro let’s get to the data!
This was a 48-week trial exploring various Eloralintide doses, mostly without titration sigh, so fetch but also two titration arms, one that was the traditional monthly increase 3mg, 6mg, 9mg and then a higher dose 6mg arm for 20 weeks, then increased to 9mg. Everything else was zero titration as seen below. Also, of note fully 50% of the 1mg group dropped out of the trial and not because of side effects. More on that in a minute.

Results reported were the usual, weight loss, cardiometabolic markers and vitals. Lilly ALSO published the full phase 2 data at the same time in The Lancet and to say there’s some interesting information is almost an understatement. Let us start with the weight loss:

Well, hello there Amylin. I didn’t respect your game. This is where my cognitive dissonance broke, I was honestly expecting about 12-14% weight loss. Instead we’re looking at tirzepatide like weight loss at maximum and 2.4mg of Wegovy at minimum. I genuinely said whoa out loud in the conference hall when these slides were presented. The 1mg group lost nearly 10% of their body weight but still 50% dropped out! What’s happening here?

Now, caveat, Lilly indicated phase 3 would include slow titration, so these results may change but the slowest titration arm still reached nearly 17% weight loss without a plateau. That matches semaglutide and given the slope would probably reach nearly 20% weight loss in a 72 week trial by itself, which would only be 2% behind CagriSema. Even broken down by category we have 1/3rd of folks of the 3/6/9mg arm lost at least 20% of their body weight. And when broken down by lean mass vs fat mass loss by DEXA scan it was similar to semaglutide 70% fat, 30% lean, which is also in line with bariatric surgery.

Moving on to cardiometabolic markers we see large drops in fasting insulin, and small but modest changes in glucose, A1c, and lipid markers consistent with weight loss. All of these are consistent with cagrilintide. It also causes a slight slowing of the heart rate, also a consistent effect seen with amylin agonists.

One last note before I get to the side effects, as noted Lilly published the full phase 2 data, which means they also published a supplemental appendix and if you’ve read this blog, you know my mantra, read the appendix and once again I found two fascinating things. One is related to the kidney.
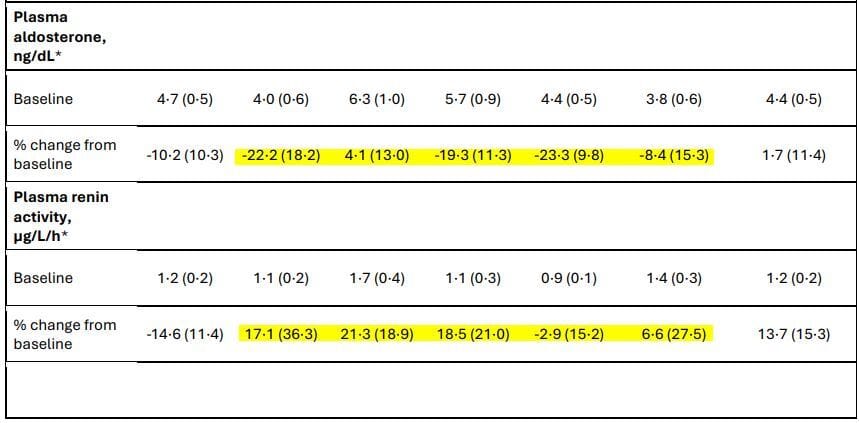
Highlights mine

Highlights again mine
It does a couple things, reducing aldosterone and increasing renin levels. Typically this would cause some minor effects on blood pressure and it does lower blood pressure by about 5mmHg systolic. But look at the GFR. It increases GFR while lowering UACR(protein in the urine.) That is a puzzling and curious finding. Amylin has receptors in the kidney so the possibility of a direct effect on the kidney is very real. This raising of GFR is also seen with glucagon agonists and is under investigation right now in multiple clinical trials. That amylin might also have beneficial renal effects plus weight loss is a very tantalizing combination. We absolutely need more data on this because Novo Nordisk has not released any kidney parameters regarding CagriSema as of mid-November 2025, however, they are actively studying it in a trial. More to come in the future.
Ok, time for side effects:
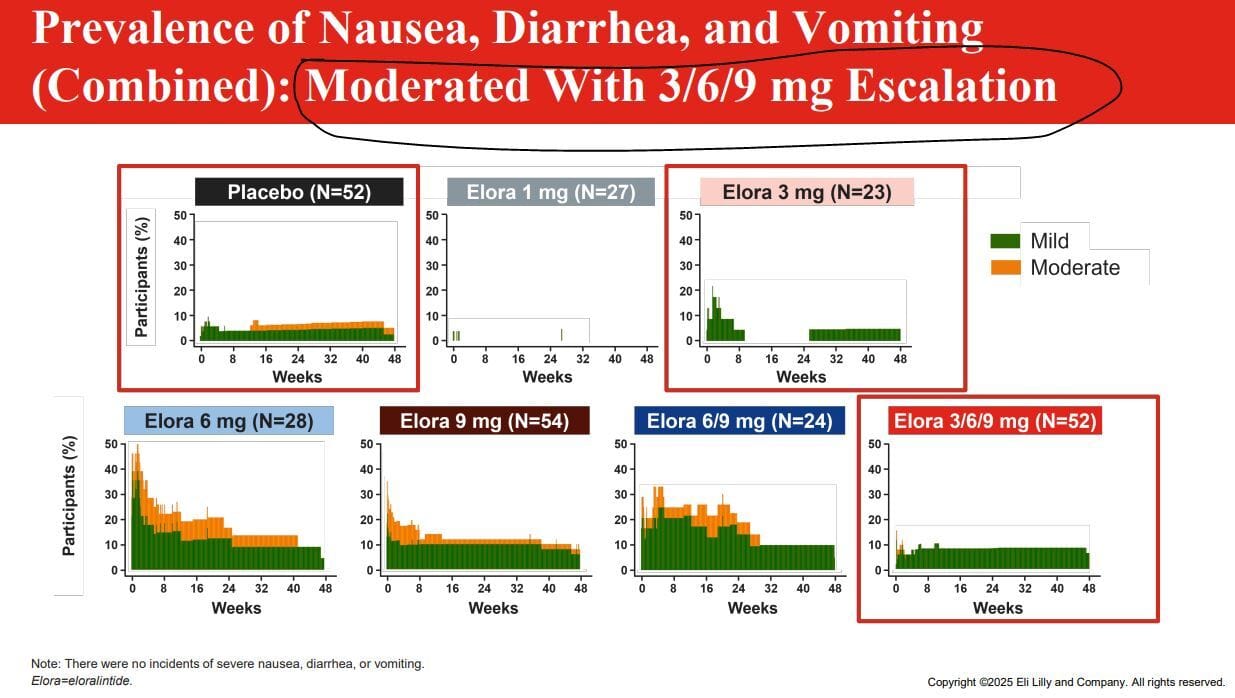


Circle and yellow highlights mine, red boxes by Lilly
Fetch didn’t happen. The high doses without titration had significantly worsened side effect profiles. I think that’s pretty obvious, but I want to point out a couple remarkable things. More people caught COVID than had nausea in the 1mg arm. They had side effects that were BETTER than the placebo arm, and 50% of people dropped out. I think, because they had such a minor reaction and slow weight loss that they dropped out. People expect to have side effects on these drugs, so they drop out at the first hint of being on placebo, those patients thought they were on a placebo, but they weren’t! A rather remarkable finding.
You’ll note the boxes that Lilly highlighted in red, the placebo arm, 3mg arm and 3/6/9mg arm all have similar rates of side effects with just slightly higher rates of nausea, fatigue and diarrhea in the 3/6/9mg arm. I expect that there will be at least one arm of the phase 3 trials Lilly will run, and I suspect they may also try a 12mg arm and probably a 6mg arm as they tend to aim for 3 approved doses for weight loss indications. Anyways, these side effects are honestly the best of the bunch compared to every other drug I examined today and probably speak to Lilly trying to find tolerability and efficacy at the same time, which is laudable!
CONTENT WARNING
Now for a little dark note/content warning. Skip ahead if you don’t wish to read about mental health issues but in the course of researching this post, I was struck by something. In both published phase 3 trials of CagriSema there was noticeable worsening of mood based on PHQ-9 scores and increased reports of suicidal ideation during both trials. While Lilly did not provide PHQ-9 scores, there were no reported incidents of depression, one report of increased anxiety and only 1 report of suicidal ideation in the 1mg arm of Eloralintide.
Obviously this a small phase 2 trial versus two large phase 3 trials but regardless it’s concerning especially for CagriSema. This may be related to the non-selective nature of Cagrilintide versus Eloralintide. The calcitonin receptor that Cagrilintide activates seems to be involved in mood and dopamine signaling in the brain, and activation of this receptor might be behind the mood changes observed in the Cagrilintide trials. Obviously we’ll have to watch for this in the phase 3 Eloralintide trials as well, but given its highly selective activity on Amylin receptors and lack of engagement on the calcitonin receptor, it may not develop into an issue at the doses Lilly is testing. Certainly this is worth watching much much closer and something that could potentially doom non-selective amylin agonists overall if it turns out to be related to the calcitonin receptor.
END CONTENT WARNING
Mazdutide, aMAZing weight loss with a caveat
Finally, this blog has talked about glucagon agonism before. Heck it is why I started the blog in the first place! And we finally have some results from a GLP-1/glucagon agonist that isn't named retatrutide. And impressive results that nearly match retatrutide, but at the price of tolerability for one of the doses. This was a phase 2 ”dose finding study” per Eli Lilly, with doses being tested 6mg, 10mg and 16mg.
However, before I even get to the slides, I’m just going to point out that the presenter of this data essentially admitted the 16mg dose was probably not going to be advanced to phase 3 due to adverse side effects. Let’s start this a little backwards, here’s the side effects of Mazdutide:
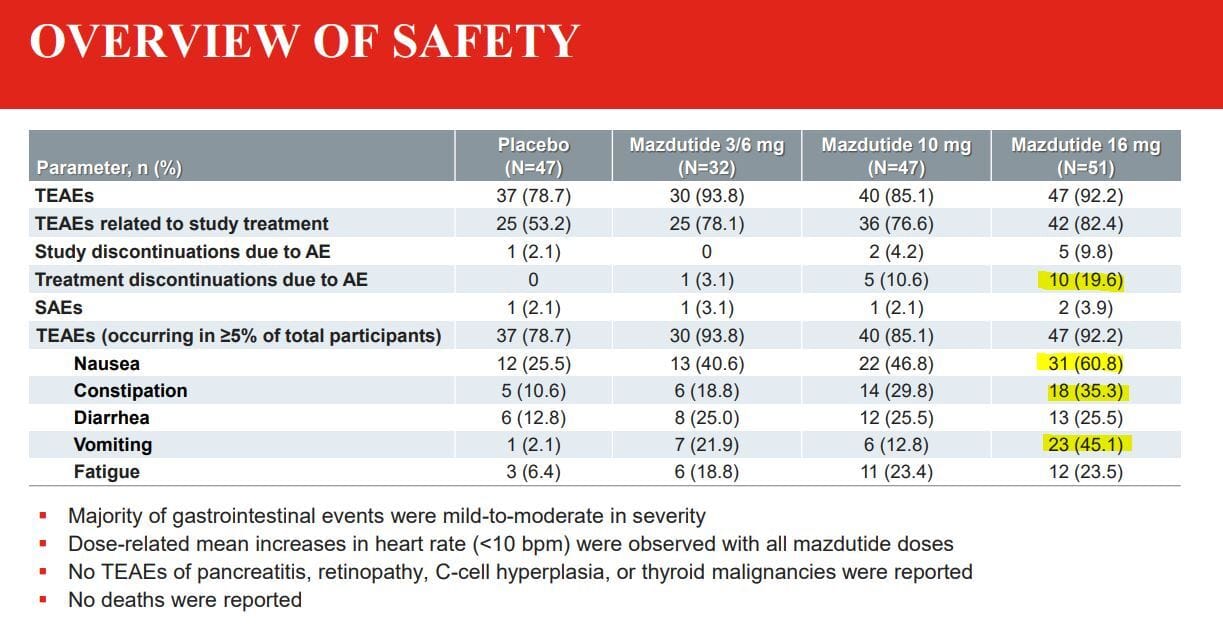
Highlights mine as always
Well, it’s pretty obvious why 16mg is a non-starter. That rate of vomiting is intense along with a super high rate of nausea and this trial used a slow titration protocol!

As you can see monthly titration except for the 6mg group, which was actually 3mg for most of the trial, then at week 32 they increased the dose to 6mg and continued on until week 48 which is a weird choice to say the least. But returning to side effects, we can see the 6mg and 10mg groups have side effect profiles comparable to other drugs we’ve looked at today and so in my mind those are both viable doses to trial in phase 3. Mazdutide is actually already approved in China for overweight, and obesity in non-diabetics at 4mg and 6mg dose. So it does work!
On the weight loss side, the 10mg dose of Mazdutide actually matches Tirzepatide 15mg in the same timeframe and the 16mg dose nearly matches Retatrutide, but as noted that appears to not be a viable dose.

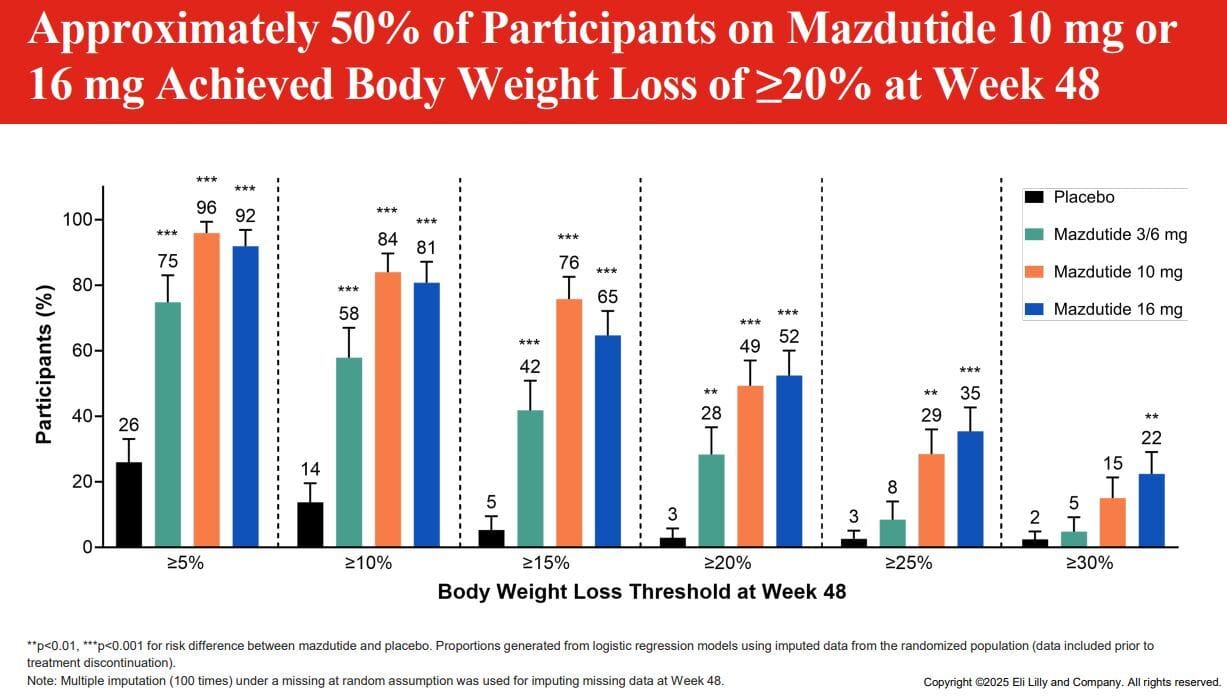
As noted, weight loss is impressive, without plateau and when broken down by categories we can see the 10mg dose is probably great, it nearly matches 16mg beat for beat until you get to the >25% weight loss category. The 3/6mg group is an odd duck and I wish they’d have just had a 6mg arm, as clearly 3mg alone rapidly plateaus off, but the 6mg kick started the weight loss again.
As far as other benefits are concerned, Lilly was thin on details as they hadn’t completed a full data analysis, but they did have A1c, glucose, triglyceride and LDL cholesterol data to share. If you’ve read my glucagon agonism article you know exactly where this is going.

There was a statistically large drop in both triglycerides and LDL cholesterol. Again, using the 10mg dose as it has the cleanest signal, about a 20% drop in LDL and a 32% drop in triglycerides. Just like retatrutide, you get weight loss with a side bonus of low-dose statin reduction in cholesterol. Moreover, despite these patients not being diabetic, A1c and fasting glucose dropped significantly consistent with the dual GLP-1/Glucagon action. This particular molecule is exciting, more proof that glucagon agonism just works even without GIP. Eli Lilly is now testing it for alcohol use disorder in a separate phase 2 trial. Phase 3 trial announcements will be made in 2026. I’ll be curious what direction Lilly intends to take with this drug.
I appreciate it if you've made it all the way to the end! All of these molecules are interesting for one reason or the other. Both HRS-9531 and MET-097 I think have a chance to help break the duopoly into smaller pieces, but it’ll still take until probably 2027 at the absolute earliest to see this happen and even then it’ll be a drug not featured during this conference. (Survodutide, dual GLP-1/Glucagon agonist from Boehringer Ingelheim) However, with that being said 3 of the molecules here are from Eli Lilly, who has basically shown a dominance of this space that I’m not sure the pharmaceutical space has ever seen. Eloralintide was their queen of the ball, with what appears to be potential best in class weight loss and remarkable tolerability. Mazdutide was a 20-minute presentation as essentially an afterthought by Eli Lilly and it arguably is better than either HRS-9531 or MET-097! Any other big pharma company would love to have just ONE of these molecules in their pipeline and Lilly presented these 3, plus they have tirzepatide, dulaglutide & retatrutide. That’s 6 molecules. And they have FIVE OTHER molecules I didn’t mention in their development pipeline in phase 1 or 2 trials. This is quickly turning into a one-horse race, with other companies scrambling to pick up the scraps off the floor at this point.
2026 will be a pivotal year for GLP-1 and Amylin medication development and approval. I'll do my best to keep this blog updated with new developments!
Up next will be GLP-1 medications and kidney disease, stay tuned for that!
References:





